KENGREAL® (cangrelor) Clinical Data
Specifically designed as an adjunct to PCI, KENGREAL has been studied in a phase III trial and in a real-world setting. Learn more below.1-3
Clinical study: CHAMPION PHOENIX
KENGREAL has a proven safety profile and has demonstrated a significant risk reduction in periprocedural thrombotic events. See how it performed vs clopidogrel in the phase III pivotal trial CHAMPION PHOENIX.1,3
Learn more about the study:
Real-world evidence: ARCANGELO
ARCANGELO assessed the safety of KENGREAL during PCI in patients with ACS in daily practice.2
ACS=acute coronary syndrome; PCI=percutaneous coronary intervention.
CHAMPION PHOENIX clinical trial data
CHAMPION PHOENIX demonstrated the efficacy and safety of KENGREAL as an adjunct to PCI. KENGREAL significantly reduced the primary composite endpoint of MACE events at 48 hours vs clopidogrel in an all-comer PCI patient population.1,3
MACE events included:
- Death
- Myocardial infarction
- Ischemia-driven revascularization
- Stent thrombosis

CHAMPION PHOENIX study design
CHAMPION PHOENIX was a randomized, double-blind, placebo-controlled, phase III trial in 11,145 patients who were undergoing either urgent or elective PCI and were receiving guideline-recommended therapy. Patients received a bolus and infusion of cangrelor or a loading dose of 600 mg or 300 mg of clopidogrel.3
22% relative risk reduction in MACE3
MACE event rate at 48 hours3
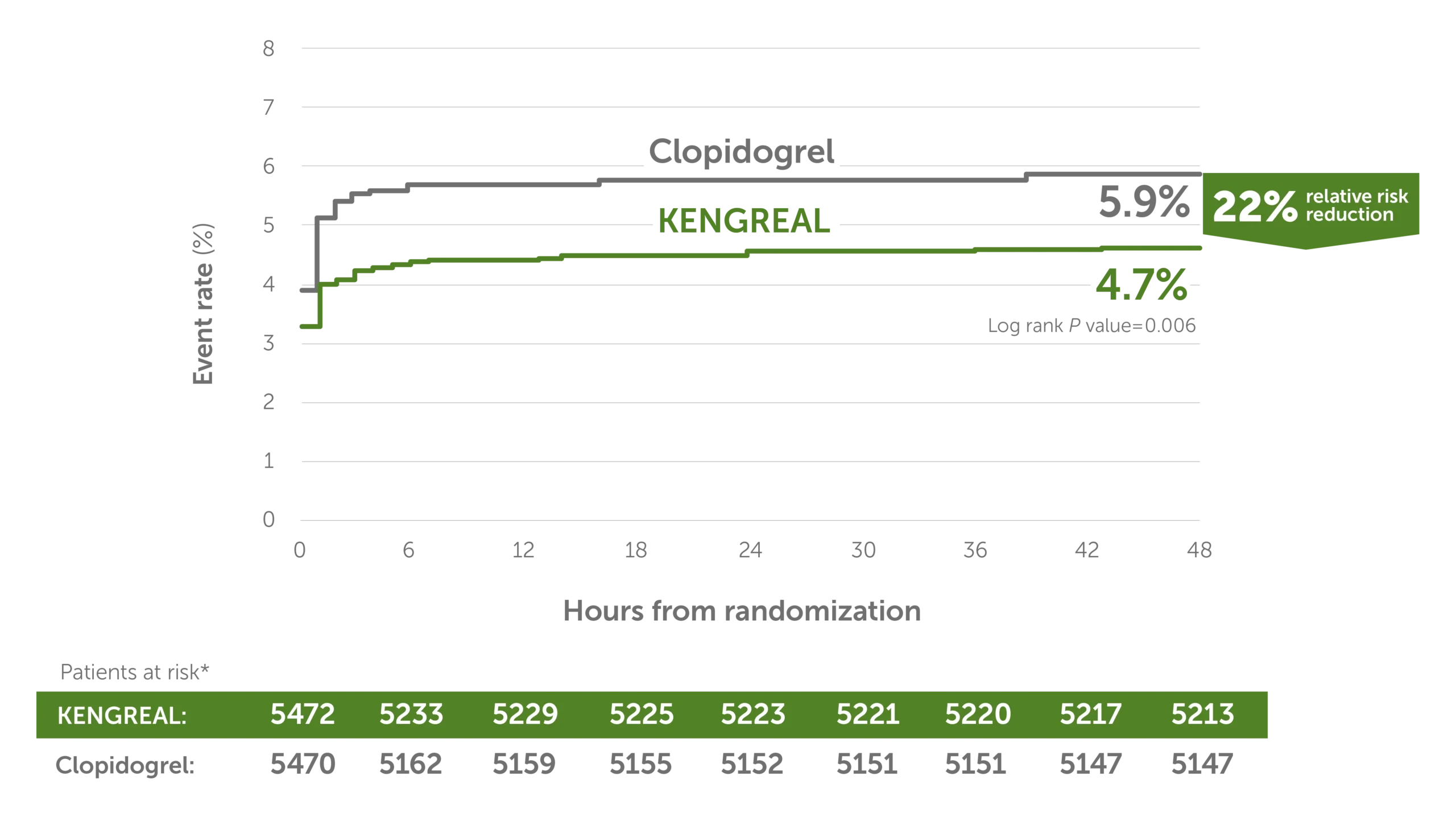
Line graph showing major adverse cardiac event rates in 6-hour increments from randomization up to 48 hours. There was a 22% relative risk reduction for KENGREAL versus clopidogrel. The event rate for KENGREAL was 4.7% and the event rate for clopidogrel was 5.9%. Patients at risk, by 6-hour increments, are as follows:
0 hour: KENGREAL 5472 / clopidogrel 5470
6 hours: KENGREAL 5233 / clopidogrel 5162
12 hours: KENGREAL 5229 / clopidogrel 5159
18 hours: KENGREAL 5225 / clopidogrel 5155
24 hours: KENGREAL 5223 / clopidogrel 5152
30 hours: KENGREAL 5221 / clopidogrel 5151
36 hours: KENGREAL 5220 / clopidogrel 5151
42 hours: KENGREAL 5217 / clopidogrel 5147
48 hours: KENGREAL 5213 / clopidogrel 5147
Note: If a subject had more than one event at 48 hours, then worst outcome counted (death > myocardial infarction > ischemia-driven revascularization > stent thrombosis).
*The mITT population is all randomized subjects who received at least one dose of study drug and underwent the index PCI procedure.
ARC=Academic Research Consortium; CI=confidence interval; CK-MB=creatinine kinase MB isoenzyme; IDR=ischemia-driven revascularization; LBBB=left bundle branch block; MACE=major adverse cardiac events; MI=myocardial infarction; mITT=modified intention-to-treat; NSTEMI=non–ST-elevation myocardial infarction; OR=odds ratio; SA=stable angina; SCAI=Society for Cardiovascular Angiography and Interventions; ST=stent thrombosis; STEMI=ST-elevation myocardial infarction; TIA=transient ischemic attack; ULN=upper limit of normal.
Outcomes at 48 hours, mITT1,3*
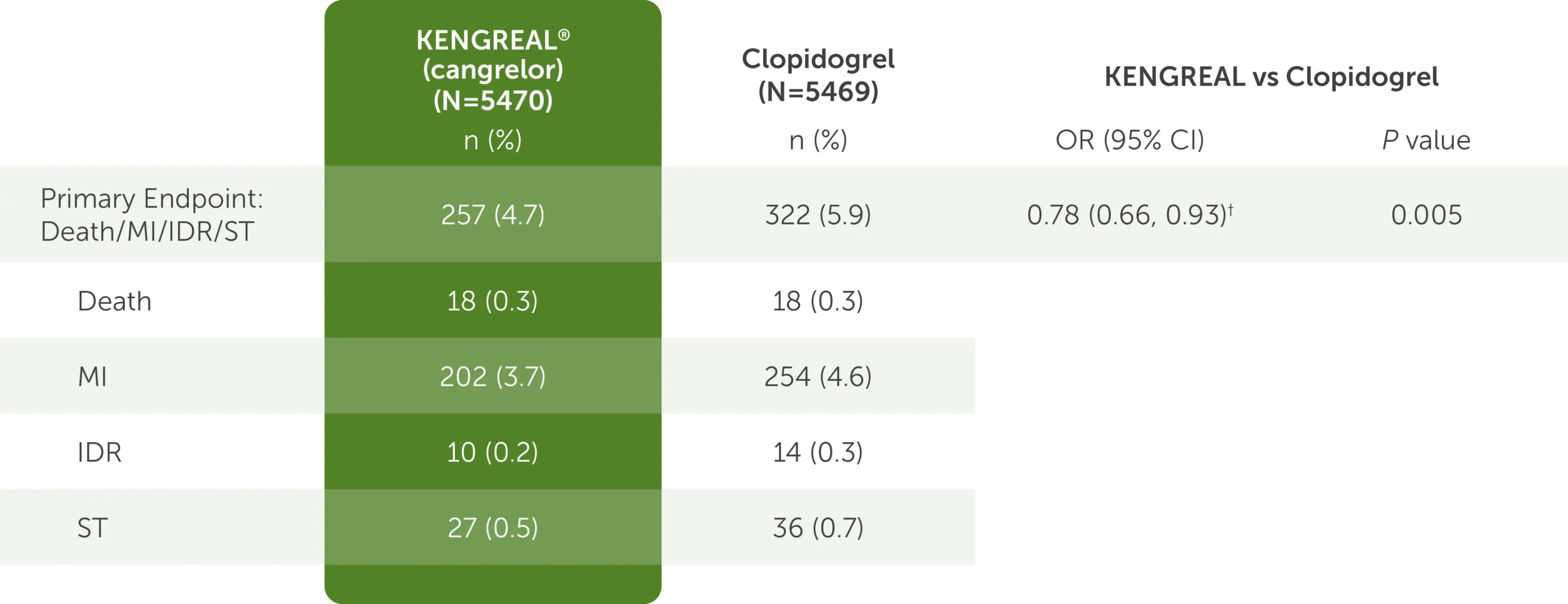
The table shows the outcomes at 48 hours for KENGREAL (N=5470) and clopidogrel (N=5469), which include the mITT primary endpoint data detailed below.
- For the composite endpoint comprised of the major adverse cardiac events of death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis, KENGREAL was 257 (4.7%) and clopidogrel was 322 (5.9%)
- For the death endpoint, KENGREAL was 18 (0.3%) and clopidogrel was 18 (0.3%)
- For the myocardial infarction endpoint, KENGREAL was 202 (3.7%) and clopidogrel was 254 (4.6%)
- For the ischemia-driven revascularization endpoint, KENGREAL was 10 (0.2%) and clopidogrel was 14 (0.3%)
- For the stent thrombosis endpoint, KENGREAL was 27 (0.5%) and clopidogrel was 36 (0.7%)
The OR for the primary endpoint was 0.78 (95% CI: 0.66, 0.93) and the P value was 0.005.
Note: If a subject had more than one event at 48 hours, then worst outcome counted (death > myocardial infarction > ischemia-driven revascularization > stent thrombosis).
*The mITT population is all randomized subjects who received at least one dose of study drug and underwent the index PCI procedure.
†Based on logistic model adjusted for loading dose and baseline patient status for primary endpoint.
ARC=Academic Research Consortium; CI=confidence interval; CK-MB=creatinine kinase MB isoenzyme; IDR=ischemia-driven revascularization; LBBB=left bundle branch block; MACE=major adverse cardiac events; MI=myocardial infarction; mITT=modified intention-to-treat; NSTEMI=non–ST-elevation myocardial infarction; OR=odds ratio; SA=stable angina; SCAI=Society for Cardiovascular Angiography and Interventions; ST=stent thrombosis; STEMI=ST-elevation myocardial infarction; TIA=transient ischemic attack; ULN=upper limit of normal.
Primary efficacy endpoint by subgroup1,3*
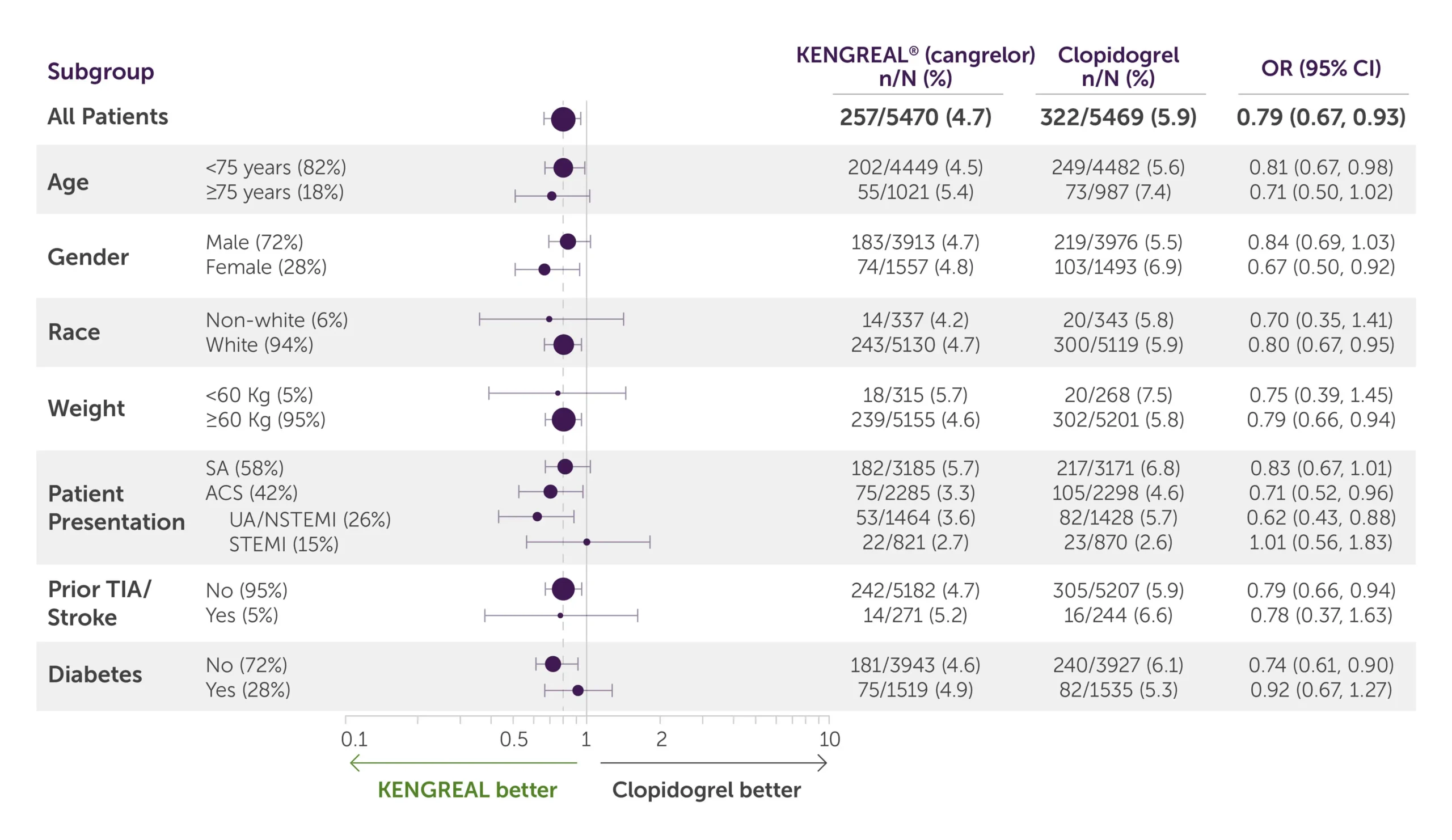
Table showing data breakdown for the primary endpoint at 48 hours according to patient subgroups of KENGREAL and clopidogrel.
All-comer population
For all-comer population, KENGREAL=257 patients with events/5470 patients (4.7%) and clopidogrel=322 events/5469 (5.9%) with an OR of 0.79 (95% CI, 0.67-0.93)
Age
- For patients less than 75 years of age (82%), KENGREAL=202 patients with events/4449 patients (4.5%) and clopidogrel=249 events/4482 with an OR of 0.81 (95% CI, 0.67-0.98)
- For patients 75 years of age and older (18%), KENGREAL=55 patients with events/1021 patients (5.4%) and clopidogrel=73 events/987 (7.4%) with an OR of 0.71 (95% CI, 0.50-1.02)
Gender
- For males (72%), KENGREAL=183 patients with events/3913 patients (4.7%) and clopidogrel=219 patients with events/3976 patients (5.5%) with an OR of 0.84 (95% CI, 0.69-1.03)
- For females (28%), KENGREAL=74 patients with events/1557 patients (4.8%) and clopidogrel=103 events/1493 (6.9%) with an OR of 0.67 (95% CI, 0.50-0.92)
Race
- For the non-white population (6%), KENGREAL=14 patients with events/337 patients (4.2%) and clopidogrel=20 events/343 (5.8%) with an OR of 0.70 (95% CI, 0.35-1.41)
- For the white population (94%), KENGREAL=243 patients with events/5130 patients (4.7%) and clopidogrel=300 patients with events/5119 patients (5.9%) with an OR of 0.80 (95% CI, 0.67-0.95)
Weight
- For patients weighing less than 60 kg (5%), KENGREAL=18 patients with events/315 patients (5.7%) and clopidogrel=20 patients with events/268 patients (7.5%) with an OR of 0.75 (95% CI, 0.39-1.45)
- For patients weighing 60 kg or more (95%), KENGREAL=239 events/5155 (4.6%) and clopidogrel=302 events/5201 (5.8%) with an OR of 0.79 (95% CI, 0.66-0.94)
Patient presentation
- For patients presenting with stable angina (58%), KENGREAL=182 patients with events/3185 patients (5.7%) and clopidogrel=217 events/3171 (6.8%) with an OR of 0.83 (95% CI, 0.67-1.01)
- For patients presenting with ACS (42%), KENGREAL=75 patients with events/2285 patients (3.3%) and clopidogrel=105 patients with events/2298 patients (4.6%) with an OR of 0.71 (95% CI, 0.52-0.96)
- For patients presenting with UA/NSTEMI (26%), KENGREAL=53 patients with events/1464 patients (3.6%) and clopidogrel=82 patients with events/1428 patients (5.7%) with an OR of 0.62 (95% CI, 0.43-0.88)
- For patients presenting with STEMI (15%), KENGREAL=22 patients with events/821 patients (2.7%) and clopidogrel=23 patients with events/870 patients (2.6%) with an OR of 1.01 (95% CI, 0.56-1.83)
Prior TIA/Stroke
- For patients with no history of TIA/stroke (95%), KENGREAL=242 patients with events/5182 patients (4.7%) and clopidogrel=305 patients with events/5207 patients (5.9%) with an OR of 0.79 (95% CI, 0.66-0.94)
- For patients with prior TIA/stroke (5%), KENGREAL=14 patients with events/271 patients (5.2%) and clopidogrel=16 patients with events/244 patients (6.6%) with an OR of 0.78 (95% CI, 0.37-1.63)
Diabetes
- For non-diabetic patients (72%), KENGREAL=181 patients with events/3943 patients (4.6%) and clopidogrel=240 patients with events/3927 patients (6.1%) with an OR of 0.74 (95% CI, 0.61-0.90)
- For patients with diabetes (28%), KENGREAL=75 patients with events/1519 patients (4.9%) and clopidogrel=82 patients with events/1535 patients (5.3%) with an OR of 0.92 (95% CI, 0.67-1.27)
Note: The figure above presents effects in various subgroups, most of which are baseline characteristics and most of which were prespecified (patient presentation and weight were not prespecified subgroups). The 95% CI numbers that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be overinterpreted.
Note: If a subject had more than one event at 48 hours, then worst outcome counted (death > myocardial infarction > ischemia-driven revascularization > stent thrombosis).
*The mITT population is all randomized subjects who received at least one dose of study drug and underwent the index PCI procedure.
ARC=Academic Research Consortium; CI=confidence interval; CK-MB=creatinine kinase MB isoenzyme; IDR=ischemia-driven revascularization; LBBB=left bundle branch block; MACE=major adverse cardiac events; MI=myocardial infarction; mITT=modified intention-to-treat; NSTEMI=non–ST-elevation myocardial infarction; OR=odds ratio; SA=stable angina; SCAI=Society for Cardiovascular Angiography and Interventions; ST=stent thrombosis; STEMI=ST-elevation myocardial infarction; TIA=transient ischemic attack; ULN=upper limit of normal.
Supplementary FDA-requested endpoint: Outcomes at 48 hours1*
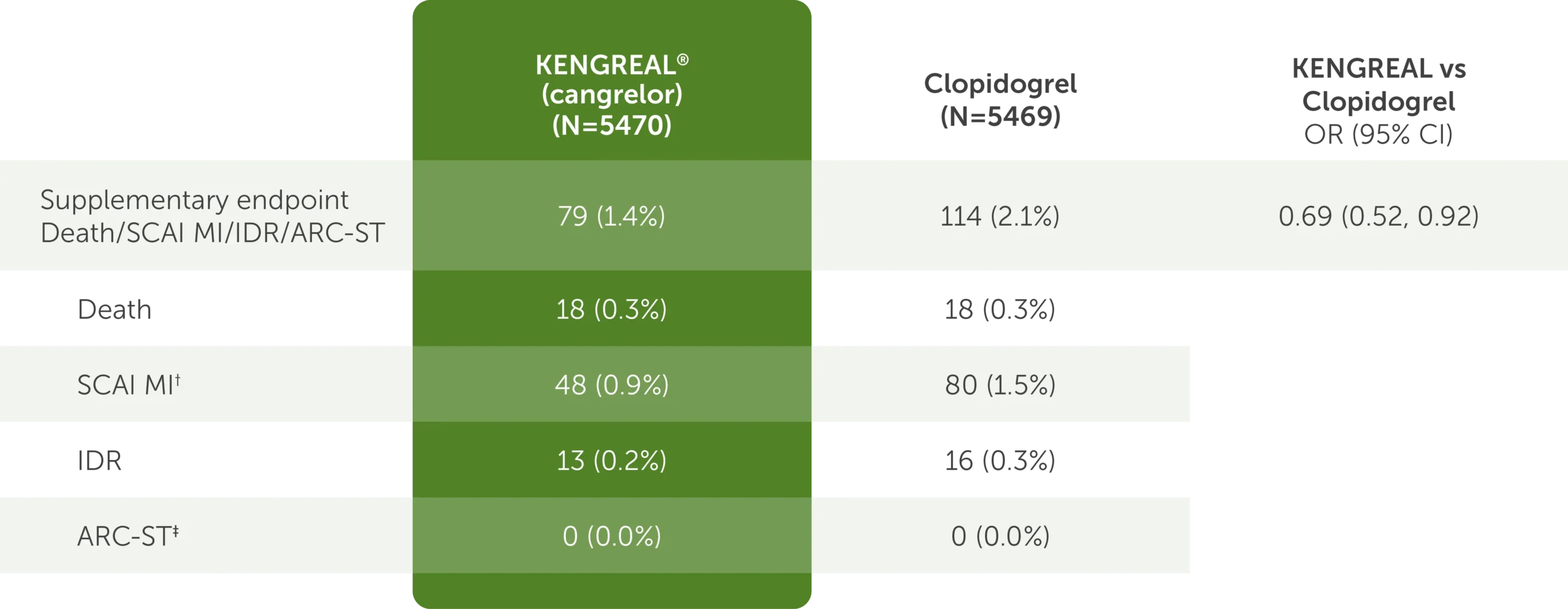
Table shows supplementary FDA-requested endpoint data of outcomes at 48 hours.1* The requested supplementary FDA-requested endpoint is defined as death, SCAI MI, IDR, and ARC-ST. For KENGREAL (cangrelor), N=5470. For clopidogrel, N=5469. For KENGREAL vs clopidogrel OR, the CI is 95%.
The table shows the following outcomes:
- Composite Death/SCAI MI/IDR/ARC-ST
- 79 (1.4%) in the KENGREAL group
- 114 (2.1%) in the clopidogrel group
- OR=0.69 (95% CI, 0.52-0.92)
- Death
- 18 (0.3%) in the KENGREAL group
- 18 (0.3%) in the clopidogrel group
- SCAI MI†
- 48 (0.9%) in the KENGREAL group
- 80 (1.5%) in the clopidogrel group
- IDR
- 13 (0.2%) in the KENGREAL group
- 16 (0.3%) in the clopidogrel group
- ARC-ST‡
- 0 (0.0%) in the KENGREAL group
- 0 (0.0%) in the clopidogrel group
This analysis omitted 2 subcomponent events from the primary endpoint of lesser clinical significance:
- Intraprocedural stent thrombosis (defined as a new or increasing thrombus within or adjacent to a deployed stent occurring during the index PCI procedure)
- MI with <10-fold increase in creatine kinase-myocardial band (CK-MB), or with <5-fold increase in CK-MB in the presence of new Q waves or new left bundle branch block (LBBB)
Note: If a subject had more than one event at 48 hours, then worst outcome counted (death > SCAI MI > IDR > ARC-ST).
*The mITT population is all randomized subjects who received at least one dose of study drug and underwent the index PCI procedure.
†SCAI MI: CK-MB≥10X ULN, or CK-MB≥5X ULN with new Q waves or new LBBB.
‡ARC-ST defined according to the ARC definition.
ARC=Academic Research Consortium; CI=confidence interval; CK-MB=creatinine kinase MB isoenzyme; IDR=ischemia-driven revascularization; LBBB=left bundle branch block; MACE=major adverse cardiac events; MI=myocardial infarction; mITT=modified intention-to-treat; NSTEMI=non–ST-elevation myocardial infarction; OR=odds ratio; SA=stable angina; SCAI=Society for Cardiovascular Angiography and Interventions; ST=stent thrombosis; STEMI=ST-elevation myocardial infarction; TIA=transient ischemic attack; ULN=upper limit of normal.
38% relative risk reduction in stent thrombosis3
Secondary endpoint: KENGREAL significantly reduced event rate for stent thrombosis at 48 hours vs clopidogrel in an all-comer PCI cohort.
Stent thrombosis event rate at 48 hours3
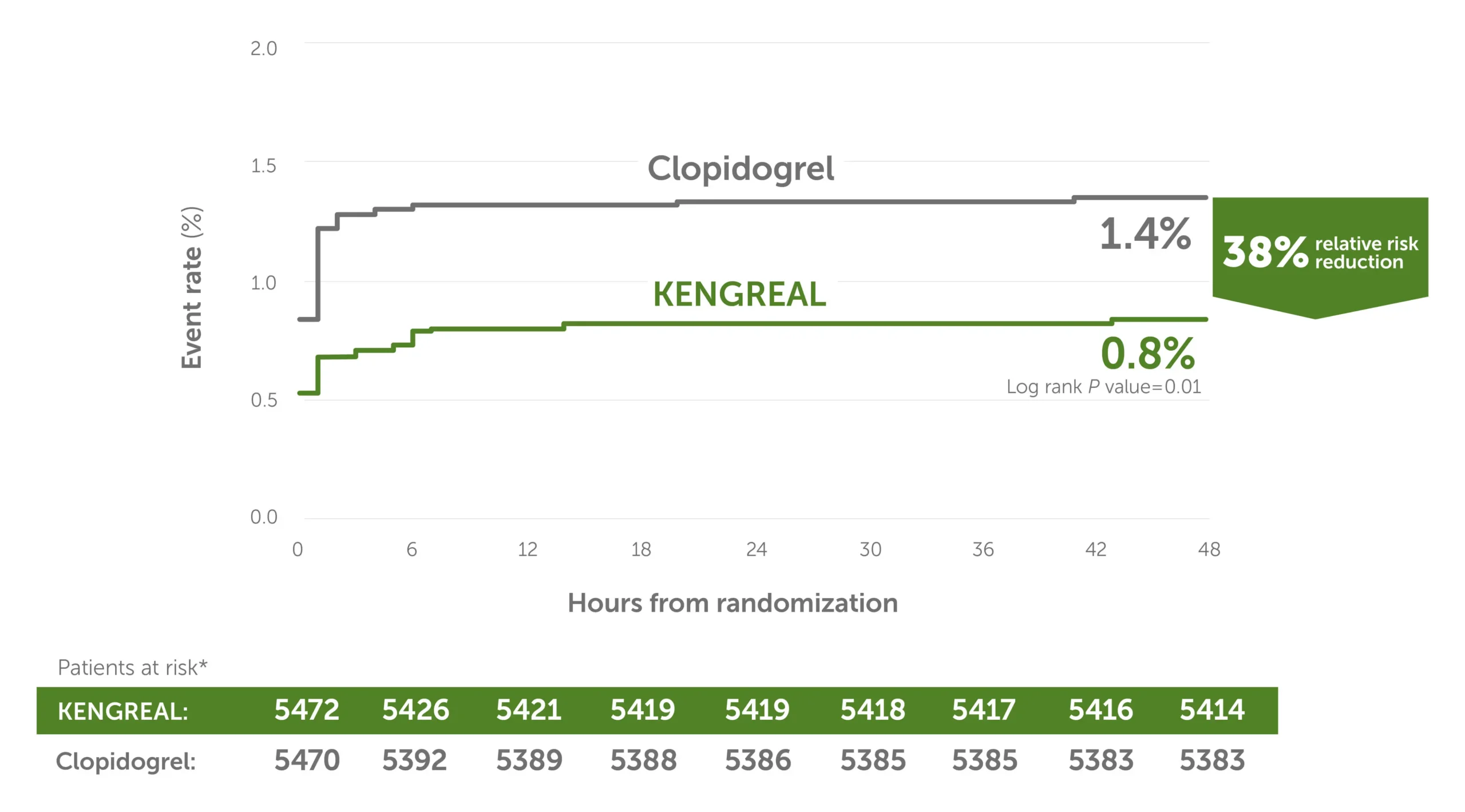
Line graph showing stent thrombosis event rates in 6-hour increments from randomization up to 48 hours. There was a 38% relative risk reduction for KENGREAL versus clopidogrel. The event rate for KENGREAL was 0.8%, and the event rate for clopidogrel was 1.4%. Patients at risk, by 6-hour increments, are as follows:
0 hour: KENGREAL 5472 / clopidogrel 5470
6 hours: KENGREAL 5426 / clopidogrel 5392
12 hours: KENGREAL 5421 / clopidogrel 5389
18 hours: KENGREAL 5419 / clopidogrel 5388
24 hours: KENGREAL 5419 / clopidogrel 5386
30 hours: KENGREAL 5418 / clopidogrel 5385
36 hours: KENGREAL 5417 / clopidogrel 5385
42 hours: KENGREAL 5416 / clopidogrel 5383
48 hours: KENGREAL 5414 / clopidogrel 5383
*The mITT population is all randomized subjects who received at least one dose of study drug and underwent the index PCI procedure.
Clinical bleeding results: CHAMPION PHOENIX
In a safety population of randomized subjects who received at least one dose of study drug1:
- Patients receiving KENGREAL had no statistically significant increase in either GUSTO-defined severe bleeding (P=0.44) or need for transfusion (P=0.16)3,7
- In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL than with clopidogrel1
- Radial access was used in only 26% of patients in the KENGREAL arm of the study4
CHAMPION PHOENIX: Bleeding events at 48 hours1
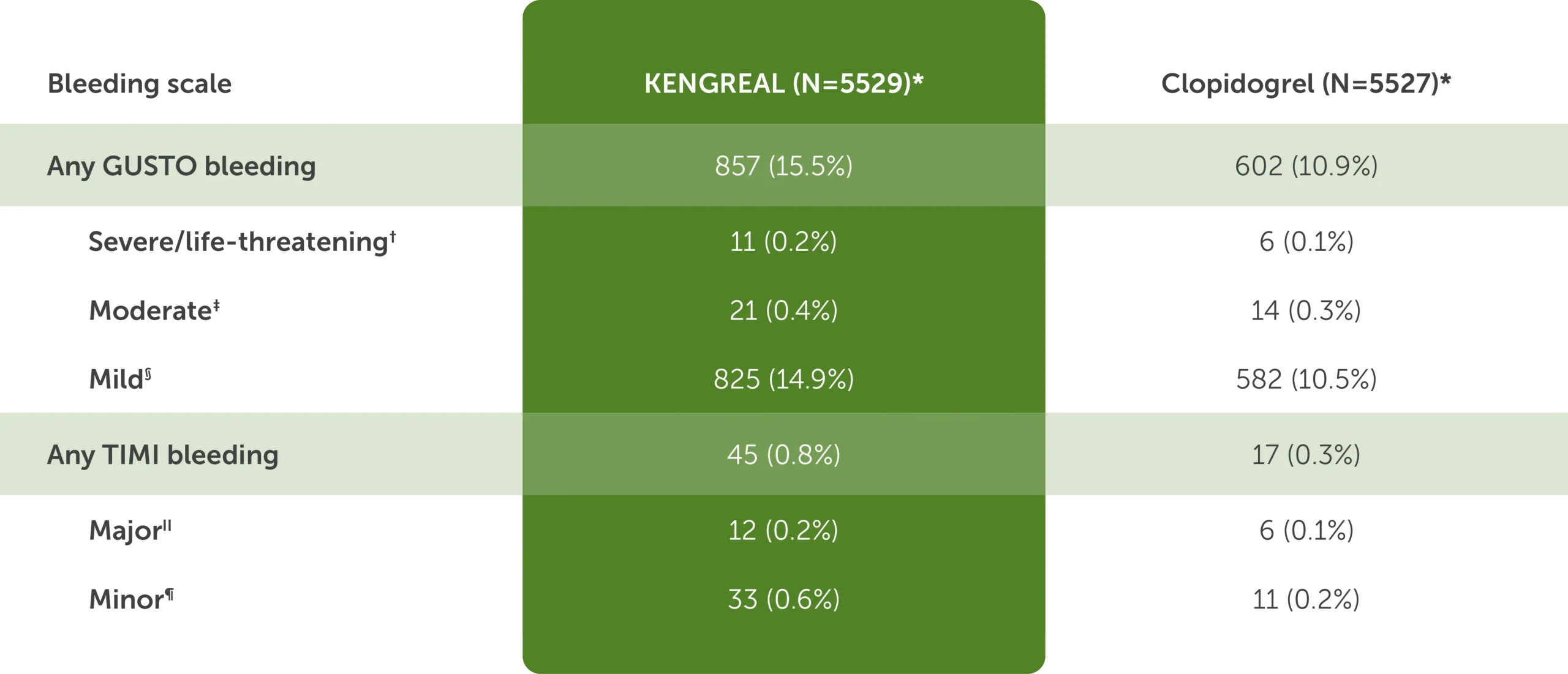
The table shows bleeding events at 48 hours in CHAMPION PHOENIX for KENGREAL (N=5529) and clopidogrel (N=5527):
- Any GUSTO bleeding: KENGREAL, 857 (15.5%) and clopidogrel, 602 (10.9%)
- Severe or life-threatening GUSTO bleeding for KENGREAL was 11 (0.2%) and for clopidogrel was 6 (0.1%)
- Moderate GUSTO bleeding for KENGREAL was 21 (0.4%) and for clopidogrel was 14 (0.3%)
- Mild GUSTO bleeding for KENGREAL was 825 (14.9%) and for clopidogrel was 582 (10.5%)
- Any TIMI bleeding: KENGREAL, 45 (0.8%) and clopidogrel, 17 (0.3%)
- Major TIMI bleeding for KENGREAL was 12 (0.2%) and for clopidogrel was 6 (0.1%)
- Minor TIMI bleeding for KENGREAL was 33 (0.6%) and for clopidogrel was 11 (0.2%)
*Safety population is all randomized subjects who received at least one dose of study drug.
†Intracranial hemorrhage or bleeding resulting in substantial hemodynamic compromise requiring treatment.
‡Requiring blood transfusion but not resulting in hemodynamic compromise.
§All other bleeding not included in severe or moderate.
‖Any intracranial hemorrhage, or any overt bleeding associated with a reduction in hemoglobin of ≥5 g/dL (or when hemoglobin is not available, an absolute reduction in hematocrit ≥15%).
¶Any overt sign of bleeding (including observation by imaging techniques) that is associated with a reduction in hemoglobin of ≥3 g/dL and <5 g/dL (or, when hemoglobin is not available, an absolute reduction in hematocrit of ≥9% and <15%).
GUSTO=GIobal Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries; TIMI=Thrombolysis in Myocardial Infarction.
51% fewer events in the first 2 hours when using KENGREAL5
A post hoc, supplemental analysis showed5:
- In the first 2 hours of PCI, during which KENGREAL was used, the combined event rate (death, SCAI MI,* ARC-ST,* and IDR) was significantly reduced vs clopidogrel (P=0.001)
- No rebound increases in cardiovascular events were observed during the period when patients were transitioning from KENGREAL to clopidogrel
Results should be interpreted with caution and with considerations of study limitations; further research may be warranted.
Time to thrombotic events5*
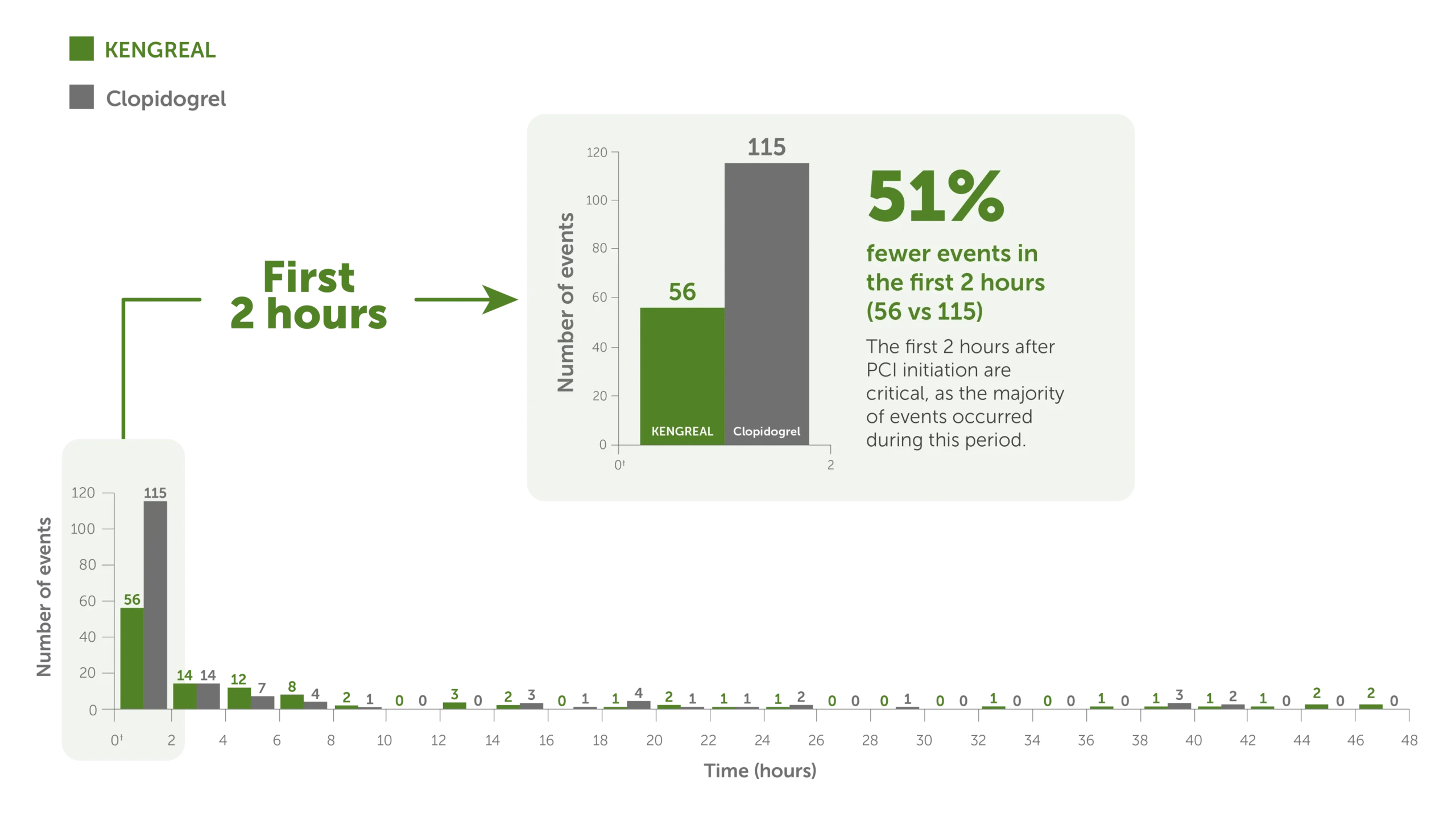
Bar graph showing 48-hour thrombotic events for KENGREAL vs clopidogrel in 2-hour increments. The event rate is markedly increased in the first 2 hours, during which there were 115 events for clopidogrel (N=5469) and 56 events for KENGREAL (N=5470). KENGREAL demonstrated 51% fewer events than clopidogrel during the first 2 hours. Number of events in 2-hour increments are as follows:
2 hours: KENGREAL 56 / clopidogrel 115
4 hours: KENGREAL 14 / clopidogrel 14
6 hours: KENGREAL 12 / clopidogrel 7
8 hours: KENGREAL 8 / clopidogrel 4
10 hours: KENGREAL 2 / clopidogrel 1
12 hours: KENGREAL 0 / clopidogrel 0
14 hours: KENGREAL 3 / clopidogrel 0
16 hours: KENGREAL 2 / clopidogrel 3
18 hours: KENGREAL 0 / clopidogrel 1
20 hours: KENGREAL 1 / clopidogrel 4
22 hours: KENGREAL 2 / clopidogrel 1
24 hours: KENGREAL 1 / clopidogrel 1
26 hours: KENGREAL 1 / clopidogrel 2
28 hours: KENGREAL 0 / clopidogrel 0
30 hours: KENGREAL 0 / clopidogrel 1
32 hours: KENGREAL 0 / clopidogrel 0
34 hours: KENGREAL 1 / clopidogrel 0
36 hours: KENGREAL 0 / clopidogrel 0
38 hours: KENGREAL 1 / clopidogrel 0
40 hours: KENGREAL 1 / clopidogrel 3
42 hours: KENGREAL 1 / clopidogrel 2
44 hours: KENGREAL 1 / clopidogrel 0
46 hours: KENGREAL 2 / clopidogrel 0
48 hours: KENGREAL 2 / clopidogrel 0
*Data derived represent a post hoc supplemental analysis in which the study was powered for superiority at the 48-hour time frame. SCAI MI: CK-MB ≥10X ULN, or CK-MB ≥5X ULN with new Q waves or new LBBB. ARC-ST defined according to the ARC definition.5
†Time 0 represents the start of PCI. KENGREAL for injection was administered at the time of PCI. Clopidogrel oral 300 mg or 600 mg was administered shortly before PCI or shortly afterward in patients randomized to clopidogrel.
Real-world evidence: ARCANGELO
ARCANGELO was conducted in Italy to assess the safety of cangrelor, based on the incidence of any hemorrhage event at 30 days post PCI (BARC criteria)2:
- Multicenter, observational, prospective cohort study
- Eligible patients (N=995) had ACS and were undergoing PCI
- All patients included in the analysis received cangrelor and transitioned to either ticagrelor (73.4%), clopidogrel (13.9%), or prasugrel (12.8%) per clinical practices
- Patients received cangrelor as a 30 mcg/kg intravenous bolus, followed immediately by a continuous intravenous infusion of 4 mcg/kg/min
- The EMA SmPC protocols followed in ARCANGELO use transition strategies that are different from those in the US; ACS treatment strategies may also vary between EU and US
- Differences in the rates of MACE and bleeding between the real-world ARCANGELO study and the CHAMPION PHOENIX pivotal trial may be attributable to advancements in PCI practices, such as improvements in device proficiency and clinician knowledge
ARCANGELO clinical bleeding results
- The study population included 597 STEMI (60%) and 398 NSTE-ACS (40%) patients2
- The BARC bleeding classification was used2
- Radial access was used in 93.1% of the PCI cases2
- Radial arterial access is associated with reduced postprocedural bleeding, fewer vascular complications, and improved outcomes vs femoral access2,6
- In 97.7% of the PCI procedures, a DES was used2
- Increased DES use reflects current guidelines
- The MACE rate was 1.4% (MACE events: death, MI, IDR, ST)2
- Patients were monitored for 30 days2
Clinical bleeding events within 30 days post PCI2
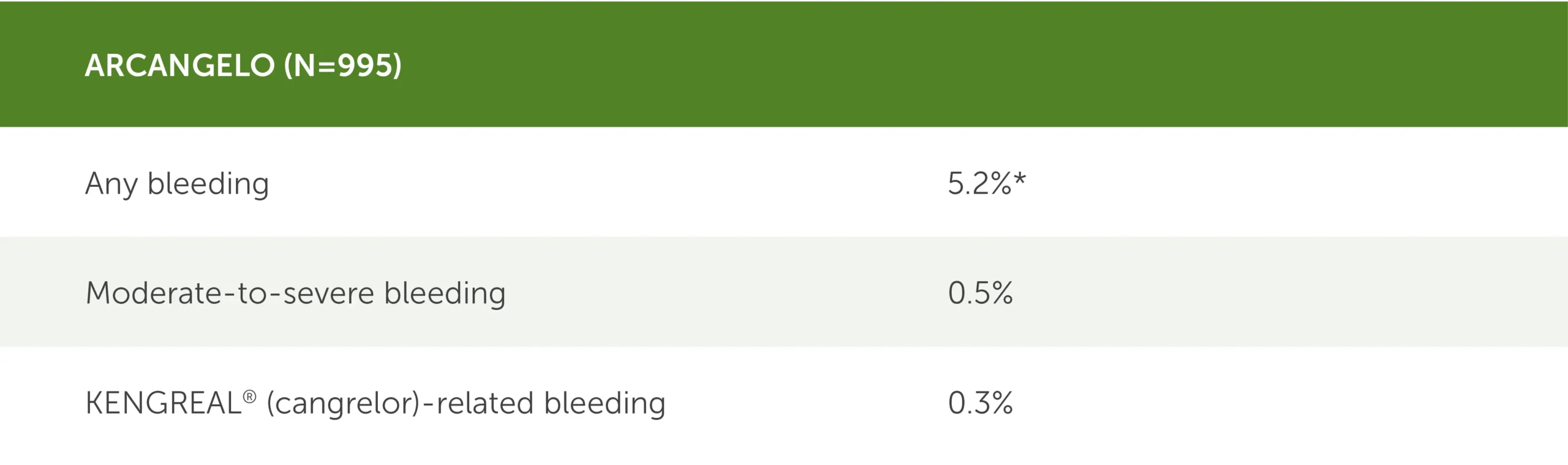
* 95% CI (3.9%-6.8%).
Table showing clinical bleeding events in the real-world ARCANGELO study (N=995) during the 30 days following PCI. BARC, or Bleeding Academic Research Consortium, was the bleeding classification used.
- Any bleeding: 5.2% (95 CI, 3.9%-6.8%)
- Moderate-to-severe bleeding: 0.5%
- KENGREAL-related bleeding: 0.3%
Note that ARCANGELO used a different bleeding classification than those used in CHAMPION PHOENIX, and the use of radial access has evolved since the CHAMPION PHOENIX trial.1-4
BARC=Bleeding Academic Research Consortium; DES=drug-eluting stent; EMA=European Medicines Agency; MI=myocardial infarcation; NSTE-ACS=non-ST-elevation acute coronary syndrome; SmPC=Summary of Product Characteristics.
Why KENGREAL when treating complex lesions?
See the efficacy data that show the benefit of using KENGREAL in high-risk PCI.
High-Risk or Complex PCIImportant Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
References: 1. KENGREAL® (cangrelor) Prescribing Information. 2022. 2. De Luca L, Calabro P, Capranzano P, et al. Safety of cangrelor and transition to oral P2Y12 inhibitors in patients undergoing percutaneous coronary intervention: the ARCANGELO study. Eur Heart J Open. 2023;3(4):oead076. 3. Bhatt DL, Stone GW, Mahaffey KW, et al; CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303-1313. 4. Gutierrez JA, Harrington RA, Blankenship JC, et al. The effect of cangrelor and access site on ischaemic and bleeding events: insights from CHAMPION PHOENIX. Eur Heart J. 2016;37(4):1122-1130. 5. Cavender MA, Bhat DL, Stone GW, et al. Consistent reduction in periprocedural myocardial infarction with cangrelor as assessed by multiple definitions findings from CHAMPION PHOENIX (cangrelor versus standard therapy to achieve optimal management of platelet inhibition). Circ Cardiovasc Interv. 2022;15:e010390. 6. Lawton JS, Tamis-Holland JE, Bangalore S. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e18-e114. 7. Jatene T, Harrington RA, Stone GW, et al. Investigator-reported bleeding
versus post hoc adjudication of bleeding: lessons from the CHAMPION
PHOENIX trial. J Am Coll Cardiol. 2016;67(5):596-598.
