KENGREAL® (cangrelor)
Dosing & Administration
Using KENGREAL for your appropriate patients begins with weight-based dosing that is independent of age and hepatic or renal function.1
How is KENGREAL dosed?
The recommended dosage of KENGREAL is a 30-mcg/kg IV bolus immediately followed by a 4-mcg/kg/min IV infusion. Be sure to administer KENGREAL via a dedicated IV line.1
Weight-based dosing in 2 steps1
Step
1
Bolus (mL):
30 mcg/kg x body weight (kg)
200 mcg/mL
- Remove the bolus dose from the IV bag, never from the reconstituted vial
- Use manual IV push or pump to administer the bolus volume in <1 minute
- Ensure the bolus is completely administered before the start of PCI
Step
2
Infusion (mL/hr):
4 mcg/kg/min x 60 min/hr x body weight (kg)
200 mcg/mL
- Begin the infusion immediately after the bolus
- Continue for at least 2 hours or the duration of the procedure, whichever is longer
Dosing table
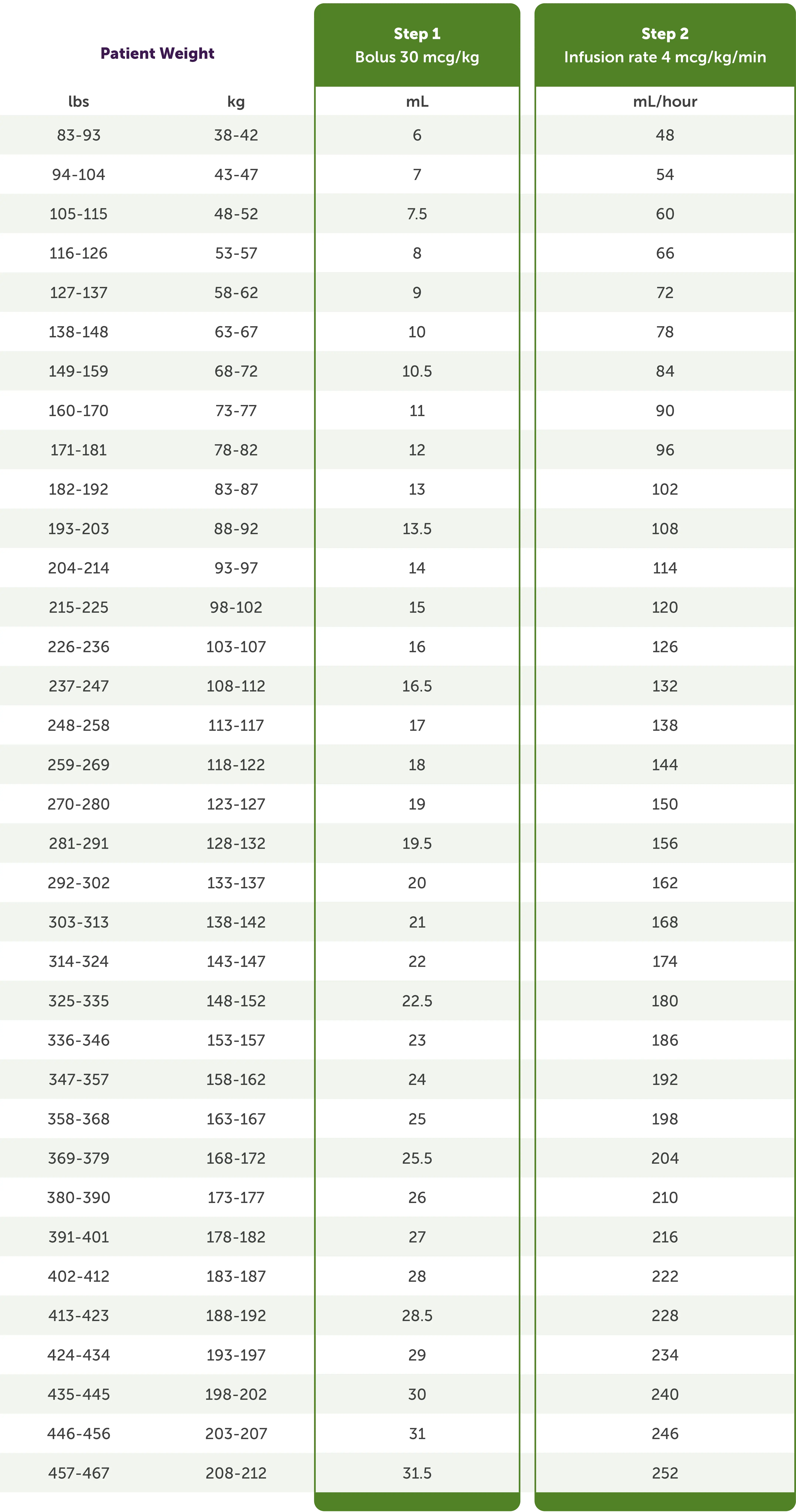
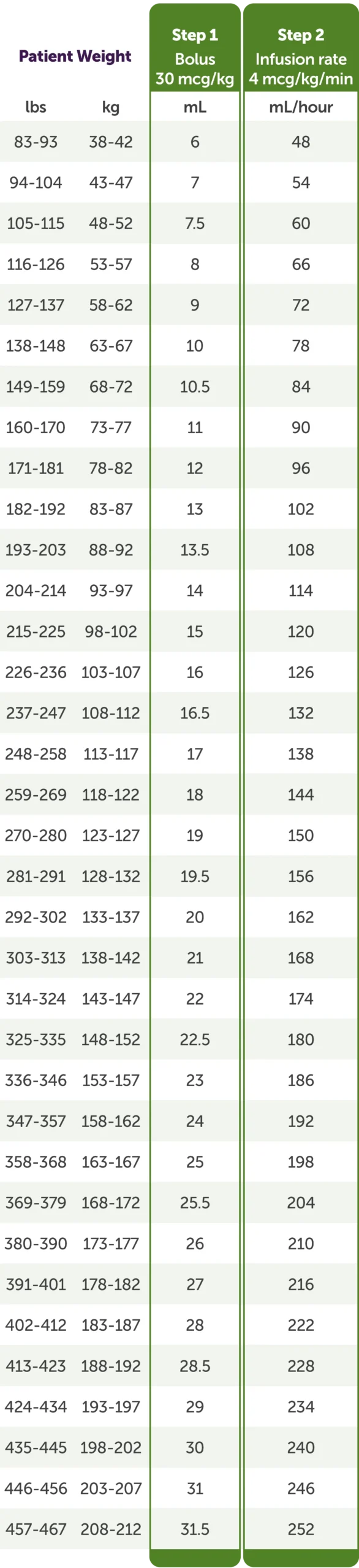
No age-based dose adjustment is required in elderly patients (≥75 years) or for patients with renal or hepatic insufficiency.
IV=intravenous; PCI=percutaneous coronary intervention.
Preparing & administering KENGREAL1
Reconstitute and dilute starting with a sterile, lyophilized powder in 10-mL, single-use vials, each containing 50 mg of KENGREAL.
Reconstitute
Reconstitute the contents of the vial immediately before dilution:
- Add 5 mL of Sterile Water for Injection to one 50-mg vial.
- Swirl gently until all material is dissolved; avoid vigorous mixing.
- Allow any foam to settle.
Reconstituted KENGREAL will be a clear, colorless to pale yellow solution. Product should not contain particulate matter.
Do not use reconstituted solution of KENGREAL without dilution.

Dilute
Dilution can be in either 0.9% Sodium Chloride Injection USP or 5% Dextrose Injection USP:
- Add contents from one reconstituted vial to one 250-mL saline or dextrose bag and mix thoroughly.
- Final concentration = 200 mcg/mL; sufficient for at least 2 hours of dosing. Patients 100 kg and over require a minimum of 2 bags.
Diluted KENGREAL is stable at room temperature for up to 12 hours in 5% dextrose injection and 24 hours in normal saline. Discard any unused portion of reconstituted solution remaining in the vial.
USP=United States Pharmacopeia.
Watch the KENGREAL prep video
See how to prepare KENGREAL for patients undergoing PCI.

Hello, my name is Tiffany. I’m a registered nurse in the adult cardiac cath lab. This video will demonstrate the procedure to prepare and administer KENGREAL, cangrelor, for injection as presented in the KENGREAL full prescribing information. Indication: KENGREAL is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention, or PCI, to reduce the risk of periprocedural myocardial infarction, MI, repeat coronary revascularization in stent thrombosis, ST, in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor. Important safety information: KENGREAL is contraindicated in patients with significant active bleeding. KENGREAL is contraindicated in patients with known hypersensitivity. Example, anaphylaxis to KENGREAL or any component of the product. Drugs that inhibit platelet P2Y12 function, including KENGREAL, increase risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL than with clopidogrel. Bleeding complications with KENGREAL were consistent across a variety of clinically important subgroups. Once KENGREAL is discontinued, there is no antiplatelet effect after an hour. The most common adverse reaction is bleeding. Instructions: use a clean surface to reconstitute KENGREAL, wash hands and don gloves. Use alcohol pad to wipe top of KENGREAL vial and port of 250-mL normal saline or dextrose 5% bag before each needle insertion. Preparation: KENGREAL is intended for IV administration as a bolus and an infusion after reconstitution and dilution. For each 50-mg vial, reconstitute by adding 5 mL of sterile water for injection. Swirl gently until all material is dissolved. Avoid vigorous mixing, allow any foam to settle, ensure that the contents of the vial are fully dissolved, and the reconstituted material is a clear, colorless to pale yellow solution. Reconstitute the vial prior to dilution in a bag. Parenteral drug products should be inspected visually for particulate matter after reconstitution. Do not use without dilution. Before administration, each reconstituted vial must be diluted further with normal saline, sodium chloride injection, 0.9% USP or 5% dextrose injection USP. Withdraw the contents from one reconstituted vial and add to one 250-mL saline or dextrose bag. Mix the bag thoroughly. This dilution will result in a concentration of 200 mcg per milliliter and should be sufficient for at least 2 hours of dosing. Patients 100 kg and over will require a minimum of 2 bags. Reconstituted KENGREAL should be diluted immediately. Diluted KENGREAL is stable for up to 12 hours in 5% dextrose injection and 24 hours in normal saline at room temperature, discard any unused portion of reconstituted solution remaining in the vial. Recommended dosing: The recommended dosage of KENGREAL is a 30 mcg per kilogram IV bolus followed immediately by a 4 mcg per kilogram per minute IV infusion. Initiate the bolus infusion prior to PCI. The maintenance infusion should ordinarily be continued for at least 2 hours or for the duration of PCI, whichever is longer. Administration of bolus and infusion: administer KENGREAL via a dedicated IV line. Step 1: bolus. Remove the bolus dose from the diluted solution, never from the reconstituted vial. Administer the bolus volume rapidly less than 1 minute from the diluted bag via manual IV push or pump. Ensure the bolus is completely administered before the start of PCI. Step 2: infusion. Begin infusion immediately after the bolus and continue for at least two hours or for the duration of PCI, whichever is longer. This concludes our presentation on the KENGREAL dosing and administration instructions. Please see the full prescribing information link on this page. Thank you for watching.
Dosing & Administration Guide
Explore dosing guidelines and instructions in this valuable clinical resource.
How to transition to an oral P2Y12 inhibitor after KENGREAL infusion
To maintain platelet inhibition after the discontinuation of KENGREAL infusion, administer 1 of the following oral P2Y12 inhibitors, as shown below.1
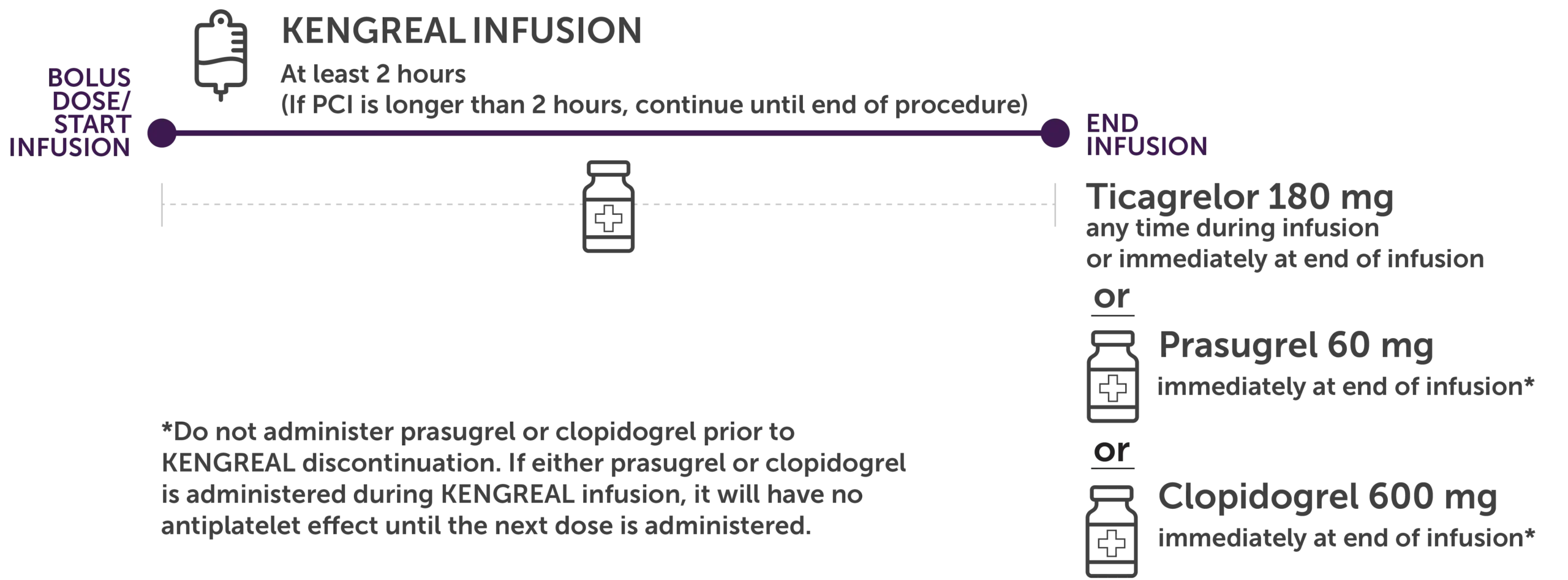
Illustration shows the timing of a KENGREAL infusion and the transition to an oral P2Y12 inhibitor. KENGREAL should be administered for at least 2 hours and longer if the procedure exceeds 2 hours. Ticagrelor 180 mg may be initiated at any time during or immediately at the end of infusion. Or prasugrel 60 mg should be initiated immediately at the end of infusion. Or clopidogrel 600 mg should be initiated immediately at the end of infusion.
Understanding the transition to an oral P2Y12 inhibitor
See how platelet inhibition is maintained while transitioning from KENGREAL to an oral P2Y12 inhibitor
The images (at right) represent a characterization of P2Y12 binding-site interactions and signaling inhibition.2-6
Ticagrelor
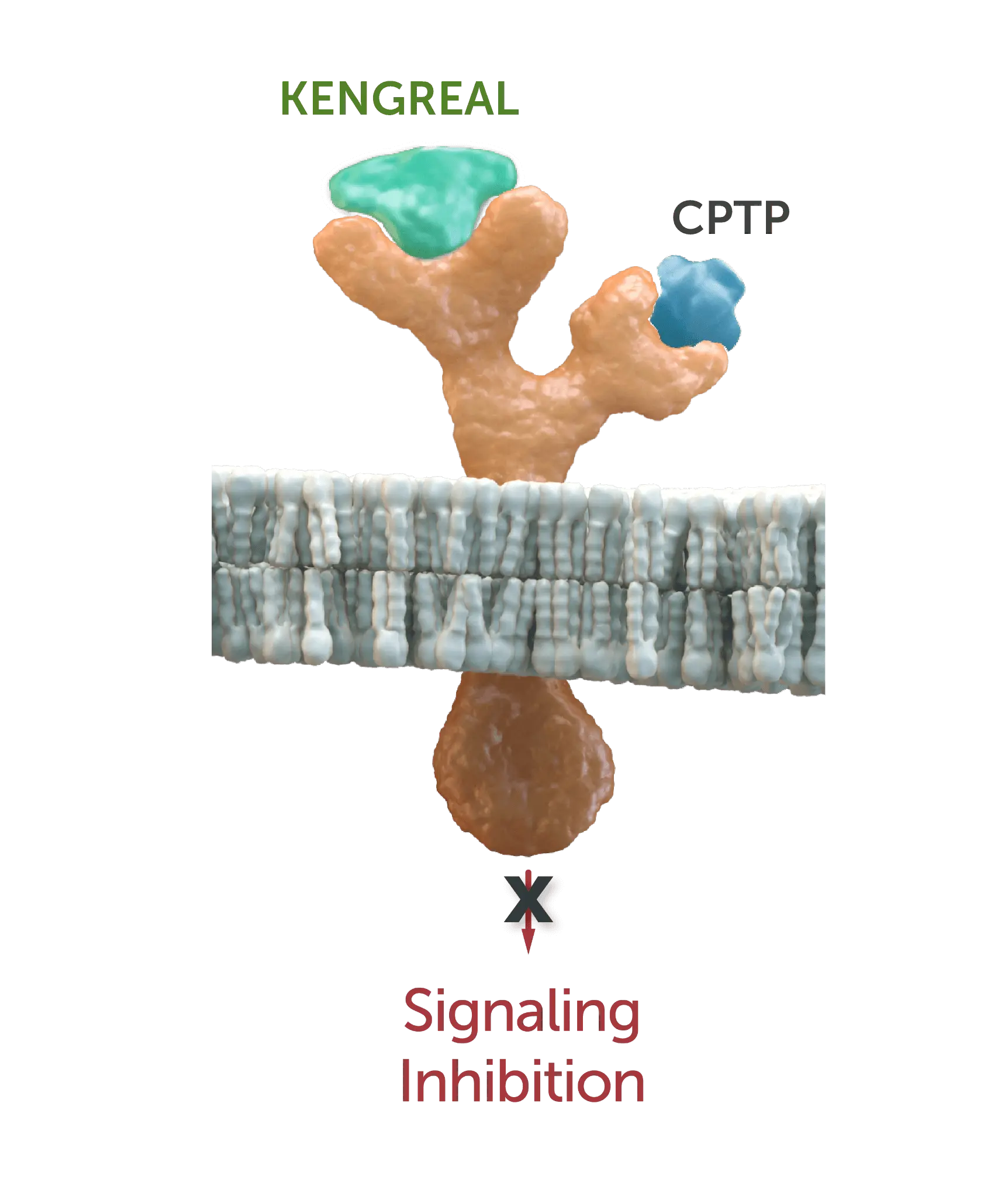
CPTP (ticagrelor) is still able to bind to P2Y12 receptor even while KENGREAL is active.2-4
Prasugrel & clopidogrel
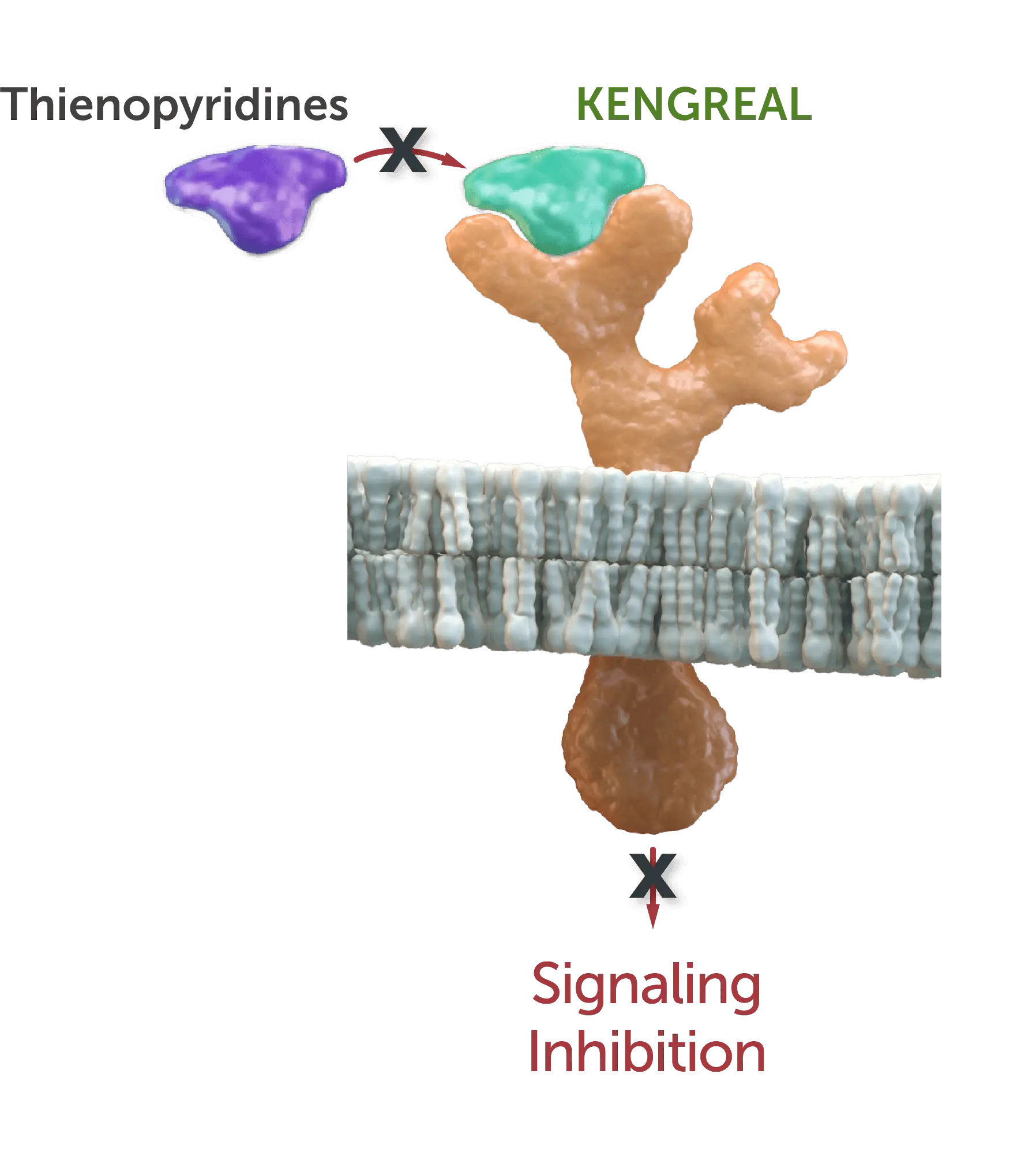
KENGREAL (cangrelor) molecule
blocks active metabolite of thienopyridines (prasugrel and clopidogrel) binding site.2-6
CPTP=cycolopentyl-triazolopyrimidine.
KENGREAL stocking & storage
You may already have KENGREAL-eligible patients in your cath lab.
Learn how to stock and store KENGREAL.
Important Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
References: 1. KENGREAL® (cangrelor) Prescribing Information. 2022. 2. Rollini F, Franchi F, Angiolillo DJ. Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nat Rev Cardiol. 2016;13(1):11-27. 3. Capodanno D, Ferreiro JL, Angiolillo DJ. Antiplatelet therapy: new pharmacological agents and changing paradigms. J Thromb Haemost. 2013;11(suppl 1):316-329. 4. Schneider DJ, Agarwal Z, Seecheran N, Keating FK, Gogo P. Pharmacodynamic effects during the transition between cangrelor and ticagrelor. JACC Cardiovasc Interv. 2014;7(4):435-442. 5. Schneider DJ, Seecheran N, Raza SS, Keating FK, Gogo P. Pharmacodynamic effects during the transition between cangrelor and prasugrel. Coron Artery Dis. 2015;26(1):42-48. 6. Schneider DJ, Agarwal Z, Seecheran N, Gogo P. Pharmacodynamic effects when clopidogrel is given before cangrelor discontinuation. J Interven Cardiol. 2015;28(5):415-419.
