Stocking KENGREAL® (cangrelor)
in the Cath Lab
Be ready for PCI with KENGREAL on hand.1
Why stock KENGREAL?
Stocking KENGREAL in the cath lab allows the bolus to be administered at the start of PCI, per the prescribing information. The maintenance infusion should then be continued for at least 2 hours or for the duration of PCI, whichever is longer.1
PCI is typically a quick procedure (median PCI <20 min in CHAMPION PHOENIX)2
KENGREAL is labeled for use at the start of PCI, and in CHAMPION PHOENIX, a significant portion of thrombotic incidents occurred within the first hour following the initiation of PCI.3
CHAMPION PHOENIX: Thrombotic events (death, MI, IDR, or ST) by hour in patients undergoing PCI3*
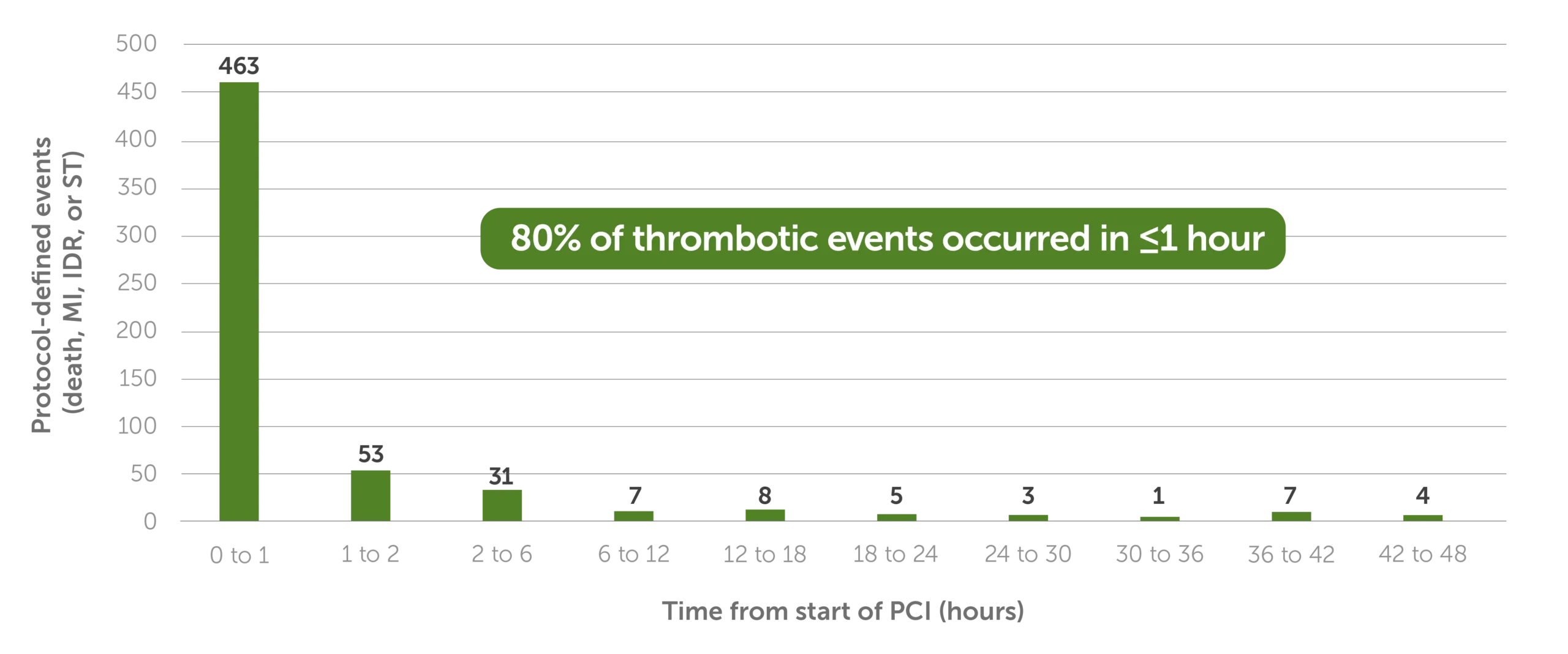
Bar graph showing the thrombotic events per hour, over the 48 hours following PCI; 80% of the thrombotic events from CHAMPION PHOENIX occurred in the first hour or less.
CHAMPION PHOENIX was a randomized, double-blind, placebo-controlled, phase III trial in 11,145 patients who were undergoing either urgent or elective PCI and were receiving guideline-recommended therapy. Patients received a bolus and infusion of cangrelor or a loading dose of 600 mg or 300 mg of clopidogrel administered shortly before PCI or shortly afterward.1,2
*Data are derived from number of patients at risk in Figure 1A of the referenced publication, which is a post hoc analysis of CHAMPION PHOENIX.3,4
IDR=ischemia-driven revascularization; MI=myocardial infarction; PCI=percutaneous coronary intervention; ST=stent thrombosis.
ACS guidelines suggest a first medical contact–to–device time of ≤90 minutes5
ACS Guidelines recommend a first medical contact–to–device time of ≤90 minutes, emphasizing the need for rapid intervention. Given its quick onset, KENGREAL may be an important consideration for PCI patients with STEMI.
KENGREAL can be prepared in the cath lab1

- KENGREAL’s preparation steps, reconstitute and dilute, can be completed right there in the cath lab
- Because KENGREAL’s clearance is independent of renal or hepatic function, no dose adjustments are needed
Clinical guideline recommendations are largely evidence based and an attempt to define practices meeting the needs of patients in most circumstances. Use of guidelines should not replace clinical judgment.
KENGREAL has not been studied or proven to impact first medical contact–to–device time.
STEMI=ST-elevation myocardial infarction.

How to store KENGREAL
Store 10-mL, single-use vials at USP Controlled Room Temperature (20°C to 25°C; 68°F to 77°F) with excursions between 15°C and 30°C (59°F and 86°F) permitted.1
Need to order KENGREAL?
Ordering is straightforward. Just click and follow the instructions.
Order KENGREALUSP=United States Pharmacopeia.
Explore additional resources
From guidelines to informational videos to educational publications, these resources allow you to visualize data and pharmacology details that put the value of KENGREAL in perspective.
Explore ResourcesImportant Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
References: 1. KENGREAL® (cangrelor) Prescribing Information. 2022. 2. Bhatt DL, Stone GW, Mahaffey KW, et al; CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303-1313. 3. Data on file. Chiesi USA, Inc. 4. Cavender MA, Harrington RA, Stone GW, et al. Ischemic events occur early in patients undergoing percutaneous coronary intervention and are reduced with cangrelor: findings from CHAMPION PHOENIX. Circ Cardiovasc Interv. 2022;15(1):e010390. 5. Rao SV, O’Donoghue ML, Ruel M, et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2025;151(13):e771-e862.
