Fewer Ischemic Events in
High-Risk or Complex PCI
Patients at higher risk for periprocedural thrombotic events benefited from KENGREAL® (cangrelor)1
For confidence in complex cases or those with high-risk lesions
In a post hoc analysis of CHAMPION PHOENIX, ~25% of patients had ≥3 angiographic high-risk lesion features.
The patients with more complex lesions had higher periprocedural thrombotic event rates, and the absolute benefit of KENGREAL increased progressively with the number of high-risk lesion features treated.1
The analysis included 9 prespecified angiographic high-risk lesion features1:
- Thrombotic lesions
- Multi-lesion PCI
- Calcified lesions
- Bifurcation lesions
- Left main lesions
- Long lesions >20 mm
- Eccentric lesions
- Tortuous lesions
- Angulated lesions
Blinded angiographic core laboratory analysis was completed in 10,854 of the 10,942 (99.2%) randomized mITT patients in CHAMPION PHOENIX (13,418 target lesions).1
PCI patients with high-risk characteristics can differ in disease severity and comorbidities, and not all high-risk lesion characteristics were accounted for in the present analysis. Treatment protocols should account for individualization of care as KENGREAL may not be appropriate for some patients. 48-hour outcomes were analyzed according to the number of prespecified angiographic high-risk lesion features treated in all patients.
Patients with more complex lesions had higher thrombotic event rates1
Number of angiographic risk features and high-risk characteristics by:
- Major adverse cardiac events
- Myocardial infarction
- Stent thrombosis
Explore the predefined primary results of CHAMPION PHOENIX.
CHAMPION PHOENIX Study48-hour event rates by number of angiographic high-risk features treated1
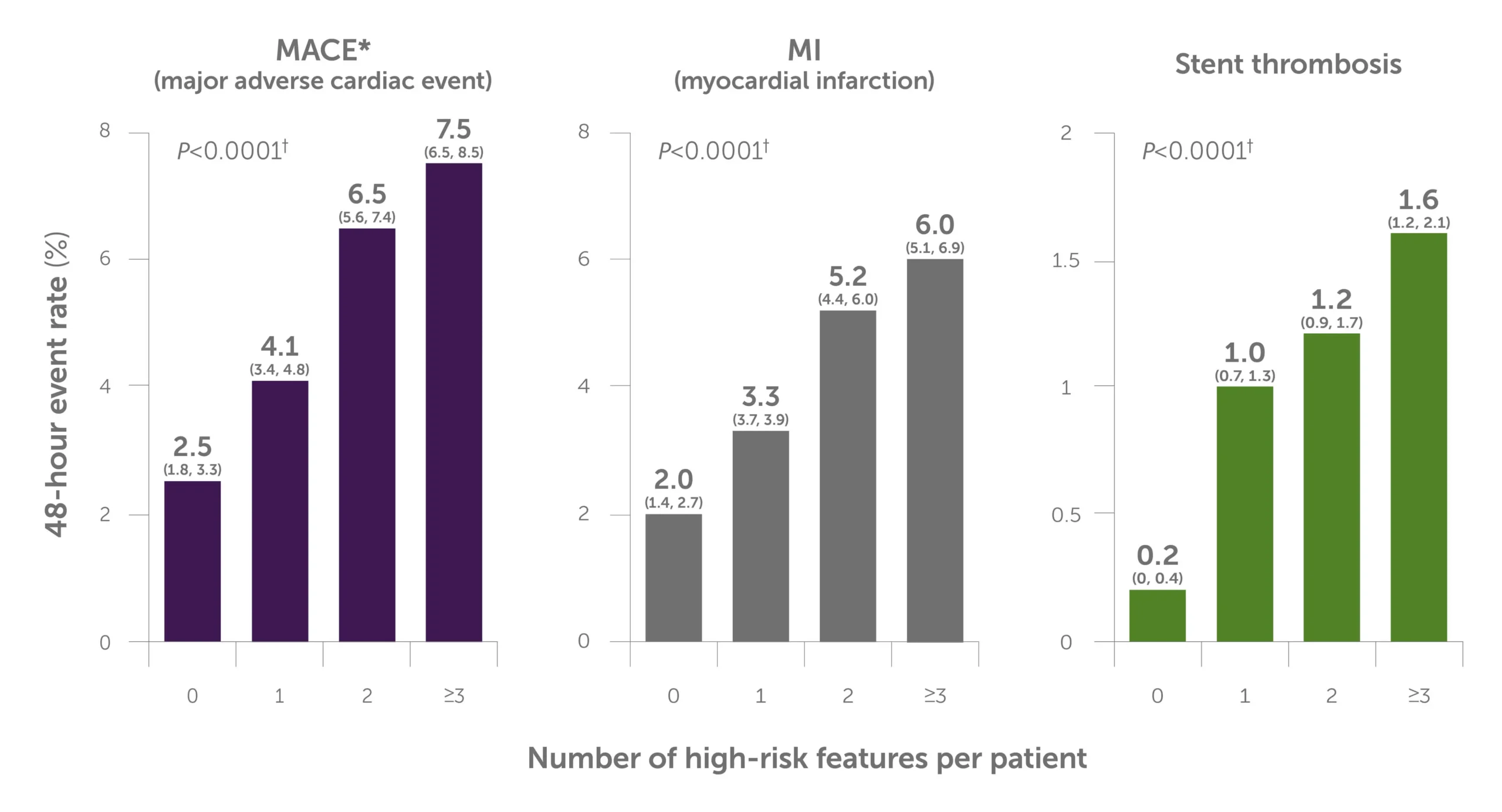
Bar graph shows how the event rate corresponds to the number of high-risk angiographic features per patient over 48 hours. The graph illustrates that the MACE, myocardial infarction, and stent thrombosis rates rose along with the number of lesions treated. For MACE, the event rate for 0 lesions was 2.5%, versus 7.5% for 3+ lesions; for myocardial infarction, the event rate for 0 lesions was 2.0%, versus 6.0% for 3+ lesions; for stent thrombosis, the event rate for 0 lesions was 0.2%, versus 1.6% for 3+ lesions.
95% confidence intervals appear under each rate estimate in parentheses.
*MACE is the measure of death, myocardial infarction, ischemia-driven revascularization, or stent thrombosis.
†Comparison between 0 and ≥3 high-risk features.
MACE=major adverse cardiac events; mITT=modified intention-to-treat; PCI=percutaneous coronary intervention.
KENGREAL’s absolute benefit increased progressively with the number of high-risk lesion features treated1
Post hoc angiographic analysis vs clopidogrel
Across patients grouped by the number of high-risk angiographic features, the 48-hour MACE rate was lower in all groups vs clopidogrel.1
48-hour MACE by number of angiographic high-risk lesion features treated1
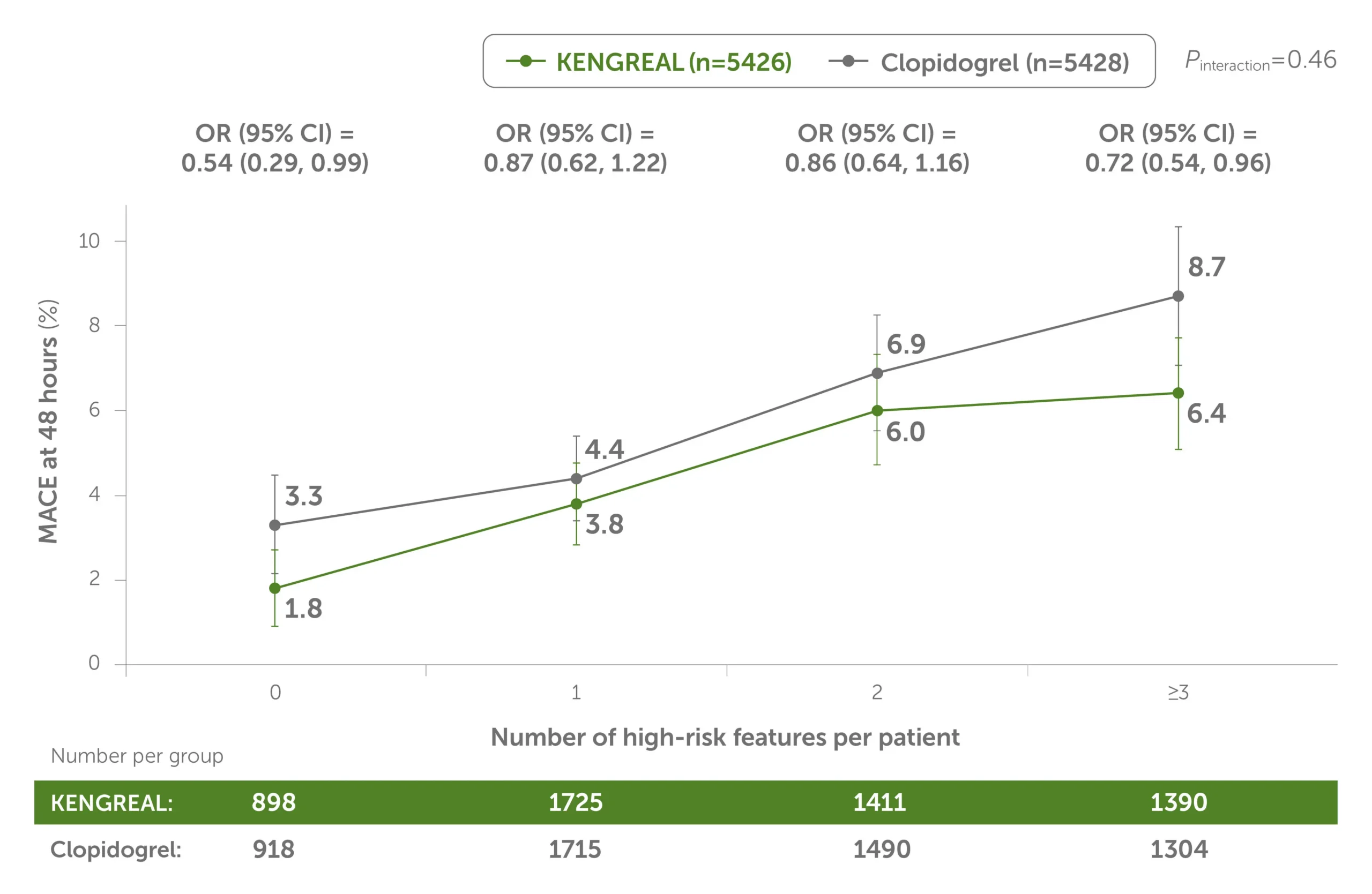
Line graph showing 48-hour MACE by number of high-risk angiographic features treated for KENGREAL vs clopidogrel. KENGREAL demonstrated a lower MACE event rate for every subset of patients, grouped by number of high-risk lesions: 0 lesions, 1 lesion, 2 lesions, and 3+ lesions. KENGREAL demonstrated event rates of 1.8%, 3.8%, 6.0%, and 6.4%, respectively, versus clopidogrel, which demonstrated respective event rates of 3.3%, 4.4%, 6.9%, and 8.7%.
This is a post hoc analysis and presents findings that supplement the predefined primary results of the CHAMPION PHOENIX trial. The CHAMPION PHOENIX trial was not designed or powered to examine the impact of KENGREAL and clopidogrel on specific angiographic risk characteristics, lesion types, and outcomes.
CI=confidence interval; OR=odds radio.
Across patients grouped by the number of high-risk angiographic features, the 48-hour ST rate was lower in all groups vs clopidogrel.1
48-hour stent thrombosis by number of angiographic high-risk lesion features treated1
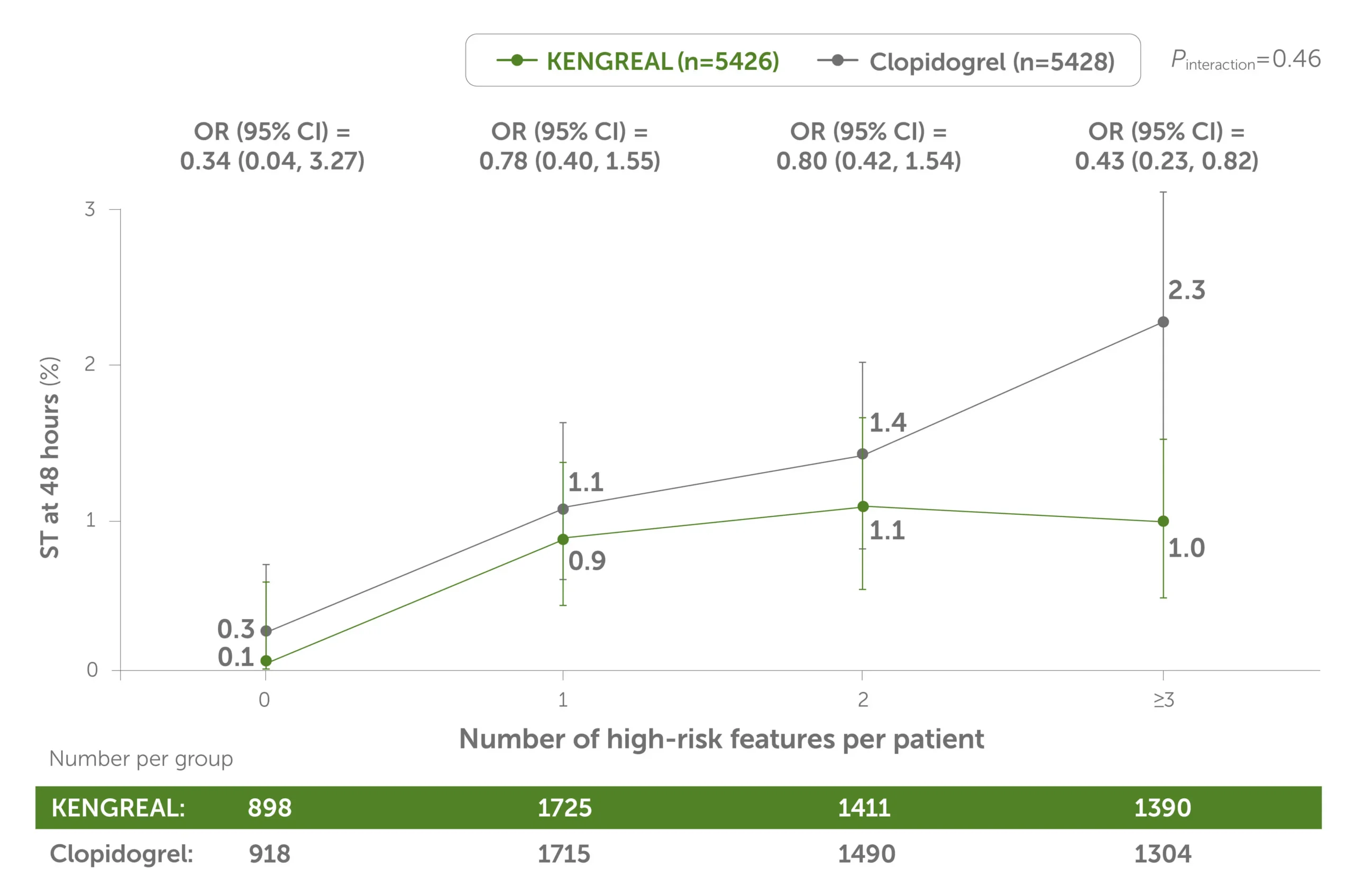
Line graph showing 48-hour stent thrombosis by number of high-risk angiographic features treated, KENGREAL vs clopidogrel. KENGREAL demonstrated a lower stent thrombosis event rate for every subset of patients, grouped by number of high-risk lesions: 0 lesions, 1 lesion, 2 lesions, and 3+ lesions. KENGREAL demonstrated event rates of 0.1%, 0.9%, 1.1%, and 1.0%, respectively, versus clopidogrel, which demonstrated respective event rates of 0.3%, 1.1%, 1.4%, and 2.3%.
This is a post hoc analysis and presents findings that supplement the predefined primary results of the CHAMPION PHOENIX trial. The CHAMPION PHOENIX trial was not designed or powered to examine the impact of KENGREAL and clopidogrel on specific angiographic risk characteristics, lesion types, and outcomes.
Which cases call for KENGREAL?
See how KENGREAL can be used clinically across varying circumstances in patients with STEMI, NSTEMI, and absorption issues.
See Use CasesNSTEMI=non–ST-elevation myocardial infarction; STEMI=ST-elevation myocardial infarction.
Important Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
Reference: 1. Stone GW, Généreux P, Harrington RA, et al. Impact of lesion complexity on peri-procedural adverse events and the benefit of potent intravenous platelet adenosine diphosphate receptor inhibition after percutaneous coronary intervention: core laboratory analysis from 10,854 patients from the CHAMPION PHOENIX trial. Eur Heart J. 2018;39(46):4112-4121.
