KENGREAL® (cangrelor) Pharmacology:
Rapid Platelet Inhibition & Offset
Prompt, potent, predictable1,2:
See how KENGREAL works.
KENGREAL is specifically designed as an adjunct to PCI1
KENGREAL is a direct P2Y12 platelet receptor inhibitor that blocks ADP-induced platelet activation and aggregation. KENGREAL binds selectively and reversibly to the P2Y12 receptor to prevent further signaling and platelet activation.1

Rapid onset
within 2 MINUTES1
Potent platelet aggregation with rapid time to maximal effect1,2

Average elimination half-life of 3-6 MINUTES1
Quick clearance via plasma metabolism enables a short half-life and prompt return to baseline platelet function1

Quick offset within
1 HOUR of discontinuation1
Rapid platelet recovery through reversible P2Y12 receptor binding,1 which may be important for patients who need CABG or surgery after PCI
ADP=adenosine diphosphate; CABG=coronary artery bypass graft; PCI=percutaneous coronary intervention.
Rapid inhibition of platelet aggregation & offset
KENGREAL is designed as a PCI adjunct1:
- Inhibits platelet aggregation within 2 minutes1,3
- Provides quick offset within 1 hour of discontinuation1
Platelet function returns to baseline within 1 hour, and with clearance independent of renal or hepatic function; no dosing adjustment is required for renal or hepatic impairment.1
KENGREAL pharmacology1,3
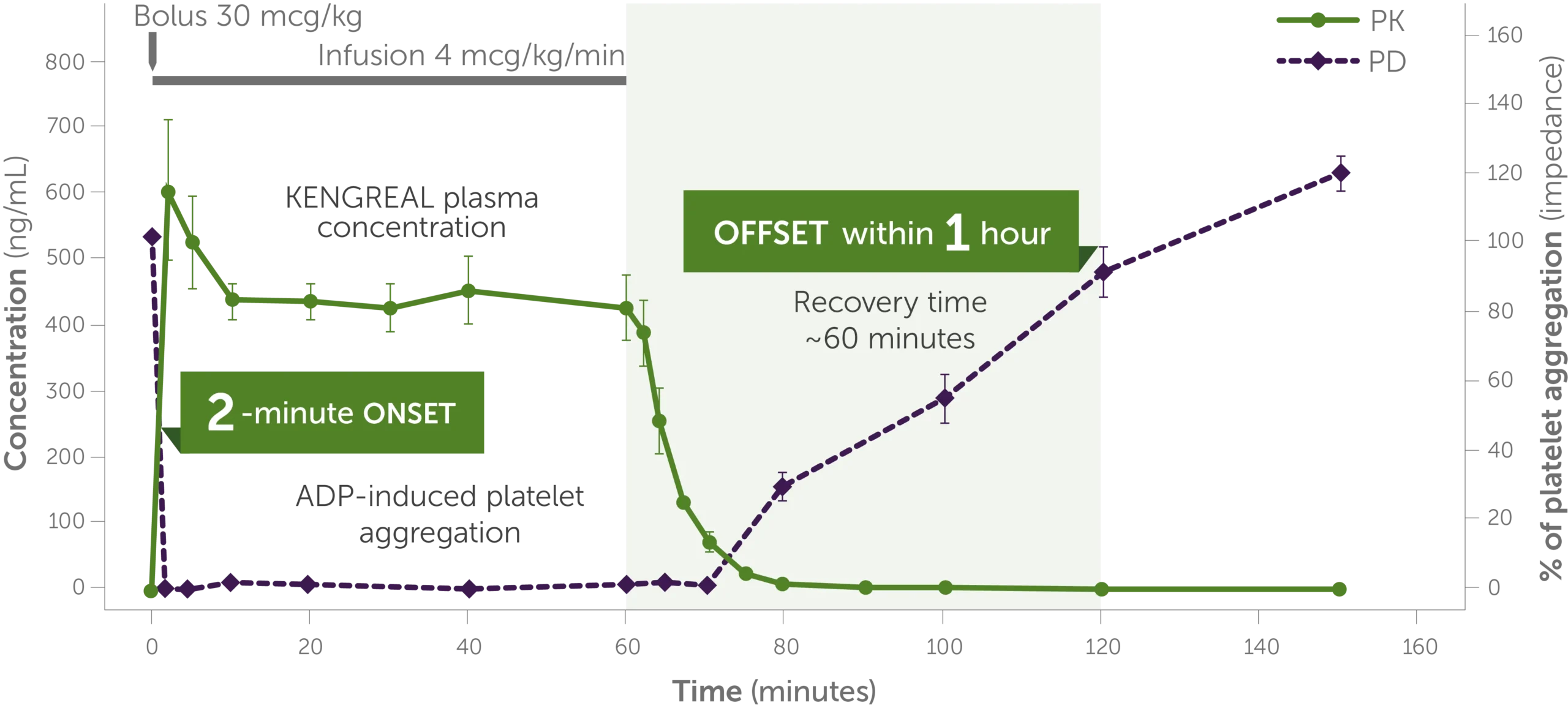
Pharmacology graph shows the trajectory of KENGREAL plasma concentration levels and ADP-induced platelet aggregation over 150 minutes, demonstrating a quick 2-minute onset and offset and recovery time within 1 hour.
Phase I study in healthy volunteers (n=9); dose: 30 mcg/kg IV bolus + 4 mcg/kg/min IV infusion. KENGREAL blood levels and platelet activity were assessed over 150 minutes by whole blood impedance aggregometry in response to 20 mcM of ADP.3
Infusion should be continued for at least 2 hours or the duration of the procedure, whichever is longer.1
IV=intravenous; PD=pharmacodynamic; PK=pharmacokinetic.
P2Y12 inhibitors come with different pharmacologic profiles
KENGREAL & oral P2Y12 inhibitors1,4-7
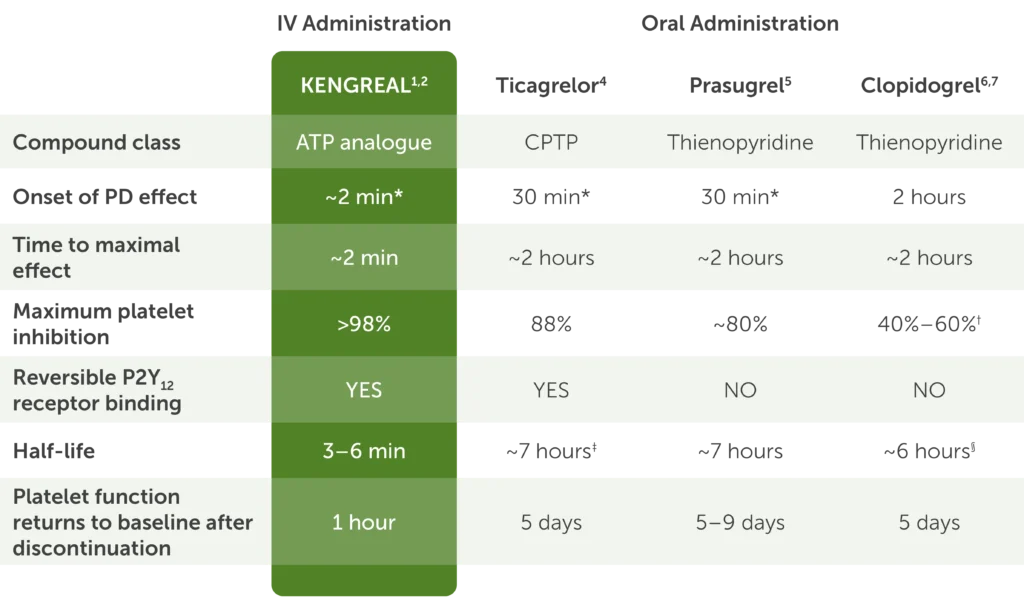
Clinical studies have not established that IV administration or pharmacologic characteristics result in superior efficacy or safety based on clinically relevant endpoints.
Table showing pharmacology of IV KENGREAL and the oral P2Y12 inhibitors ticagrelor, prasugrel, and clopidogrel with the following data:
- Compound class: KENGREAL is an ATP analogue, ticagrelor is CPTP, and prasugrel and clopidogrel are both thienopyridines
- Onset of PD effect: KENGREAL is approximately 2 minutes, ticagrelor and prasugrel are both 30 minutes, and clopidogrel is 2 hours
- Time to maximal effect: KENGREAL is approximately 2 minutes and ticagrelor, prasugrel, and clopidogrel are each approximately 2 hours
- Maximum platelet inhibition: greater than 98% for KENGREAL, 88% for ticagrelor, around 80% for prasugrel, and 40%-60% for clopidogrel
- P2Y12 receptor binding is reversible for KENGREAL and ticagrelor but not for prasugrel and clopidogrel
- Half-life is 3-6 minutes for KENGREAL, around 7 hours for ticagrelor and prasugrel, and around 6 hours for clopidogrel
- The amount of time in which platelet function returns to baseline after discontinuation is 1 hour for KENGREAL, 5 days for ticagrelor, 5-9 days for prasugrel, and 5 days for clopidogrel
*First time point at which inhibition of platelet aggregation was measured.
†40%-60% refers to average inhibition at steady state.
‡Active metabolite of ticagrelor has a half-life of 9 hours.
§Active metabolite of clopidogrel has a half-life of 30 minutes.
ATP=adenosine-triphosphate; CPTP=cyclopentyl-triazolol-pyrimidine.
When time is of the essence, KENGREAL is an effective adjunct to PCI.
See Use CasesKENGREAL inhibition of thrombus formation in vivo
In this 30-second video, see the effect of KENGREAL on the thrombotic response to laser-induced injury in the mouse cremaster muscle arteriole.2,8
Contact us for more information
Request a Rep
Note that the information featured includes in vivo data, the clinical significance of which has not been established.
The video shows the thrombotic response in a mouse treated with KENGREAL versus control. The left side shows the control response in real time and the rapid formation of platelet-rich thrombus. On the right side, the same experiment is performed, with KENGREAL introduced into the system. With KENGREAL, initial hemostasis is achieved, and we don’t see the formation of a fully occluding thrombus.
KENGREAL PD in STEMI
See the CANTIC study evaluating the PD effects of cangrelor plus crushed ticagrelor versus crushed ticagrelor alone in STEMI patients undergoing primary PCI9
STEMI=ST-elevation myocardial infarction.
Important Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
References: 1. KENGREAL® (cangrelor) Prescribing Information. 2022. 2. Data on File. Chiesi USA, Inc. 3. Akers WS, Oh JJ, Oestreich JH, et al. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2y12 receptor antagonist. J Clin Pharmacol. 2010;50(1):27-35. 4. BRILINTA® (ticagrelor) Prescribing Information. 2024. 5. EFFIENT® (prasugrel) Prescribing Information. 2022. 6. PLAVIX® (clopidogrel) Prescribing Information. 2022. 7. Hochholzer W, Trenk D, Furndi D, et al. Time dependence of platelet inhibition after a 600-mg loading dose of clopidogrel in a large, unselected cohort of candidate for percutaneous coronary intervention. Circulation. 2005;111(20):2560-2564. 8. Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875-1885. 9. Franchi F, Rollini F, Rivas A, et al. Platelet inhibition with cangrelor and crush ticagrelor in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. 2019;139(14):1661-1670.
