KENGREAL® (cangrelor) for an NSTEMI Patient With High-Risk Anatomy
Discover why KENGREAL could be a key consideration in early invasive strategy.1,2
NSTEMI patient with lesion complexity
Not an actual patient—symptoms will vary; individual clinical evaluation should be conducted to determine the best course of therapy.
Presentation
- 76-year-old female developed dyspnea, epigastric discomfort, dizziness, and nausea while gardening
- Experienced shortness of breath during daily dog walking over the past 2 weeks
- Experienced stuttering discomfort at rest accompanied by labile T-wave abnormality in the anterior leads
Medical history
- History of recent GI bleed; drank 2 glasses of wine daily
- High risk for bypass, refuses surgery
- Type 2 diabetes mellitus, last A1c 8.2%
- Hyperlipidemia
- Height 160 cm; weight 72.4 kg; BMI 28
Exam and lab values
- Troponin elevated, 2x ULN
- Chest pain despite medical management
- Pulse 92 bpm; BP 152/82 mmHg; respiratory rate 14 breaths per minute
Antiplatelet history—No antiplatelet preload
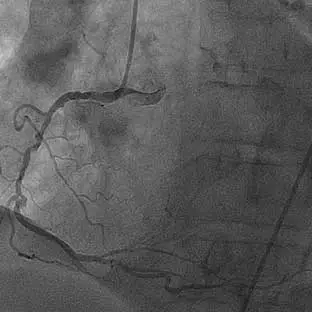
80% occlusion of the proximal LAD with evidence of calcification
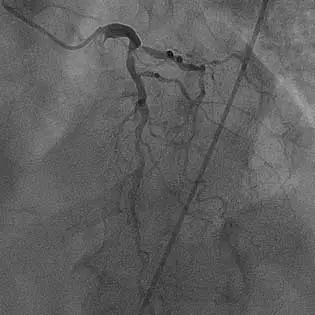
Bifurcated lesion involving the circumflex artery with evidence of calcification
BMI=body mass index; BP=blood pressure; bpm=beats per minute; GI=gastrointestinal; LAD=left anterior descending artery; NSTEMI=non–ST-elevation myocardial infarction; PCI=percutaneous coronary intervention; ULN=upper limit of normal.
KENGREAL for a variety of patient scenarios
See other KENGREAL use cases.
STEMI Case Profile Emergent Case Profile See Use CasesImportant Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
References: 1. KENGREAL® (cangrelor) Prescribing Information. 2022. 2. Bhatt DL, Stone GW, Mahaffey KW, et al; CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303-1313.

