KENGREAL® (cangrelor) for High-Risk STEMI
When time is of the essence, call on KENGREAL.1
STEMI patient with morphine coadministration
Not an actual patient—symptoms will vary; individual clinical evaluation should be conducted to determine the best course of therapy.
Presentation
- 62-year-old male arrived at hospital via ambulance; patient vomited en route
- Acute onset of crushing chest pain occurred 60 minutes ago
- Morphine administered by EMS
Medical history
- Hyperlipidemia
- Smokes 1 pack of cigarettes per day
- Height 165 cm; weight 93.0 kg; BMI 34
Exam and lab values
- Pulse 98 bpm; BP 148/94 mmHg
- Respiratory rate 22 breaths per minute
Antiplatelet history
- 81 mg aspirin per day
- No antiplatelet preload
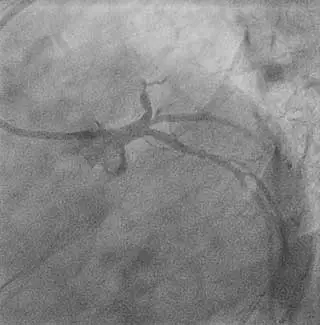
Evidence of
fresh thrombus
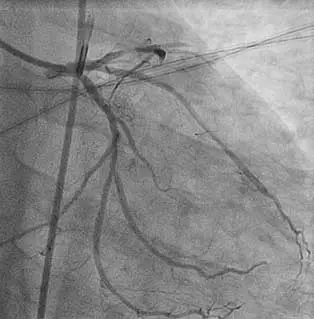
Thrombotic total occlusion
of the mid LAD
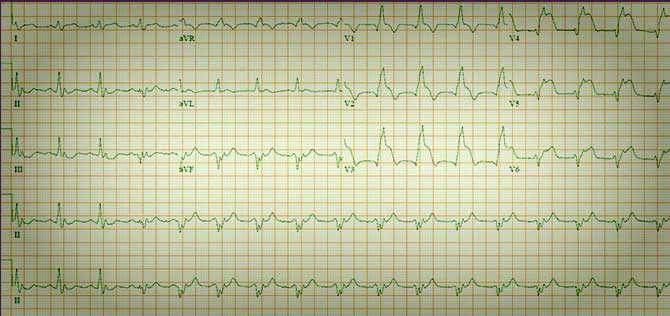
5 mm of ST elevation in anterior leads
BMI=body mass index; BP=blood pressure; bpm=beats per minute; EMS=emergency medical services; LAD=left anterior descending artery; PD=pharmacodynamics; STEMI=ST-elevation myocardial infarction.
KENGREAL for a variety of patient scenarios
See other KENGREAL use cases.
NSTEMI Case Profile Emergent Case Profile See Use CasesImportant Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
References: 1. KENGREAL® (cangrelor) Prescribing Information. 2022. 2. Data on File. Chiesi USA, Inc. 3. De Luca L, Steg PG, Bhatt DL, Capodanno D, Angiolillo DJ. Cangrelor: clinical data, contemporary use, and future perspectives. J Am Heart Assoc. 2021;10(13):e022125.

