KENGREAL® (cangrelor) Pharmacodynamics in STEMI
CANTIC assessed the comparative pharmacodynamic effects of cangrelor plus crushed ticagrelor versus crushed ticagrelor alone in STEMI patients, and it evaluated the potential for drug-drug interactions when transitioning from KENGREAL to ticagrelor in PCI (n=50; PD population=44).1
KENGREAL leads to more rapid & potent P2Y12 inhibition
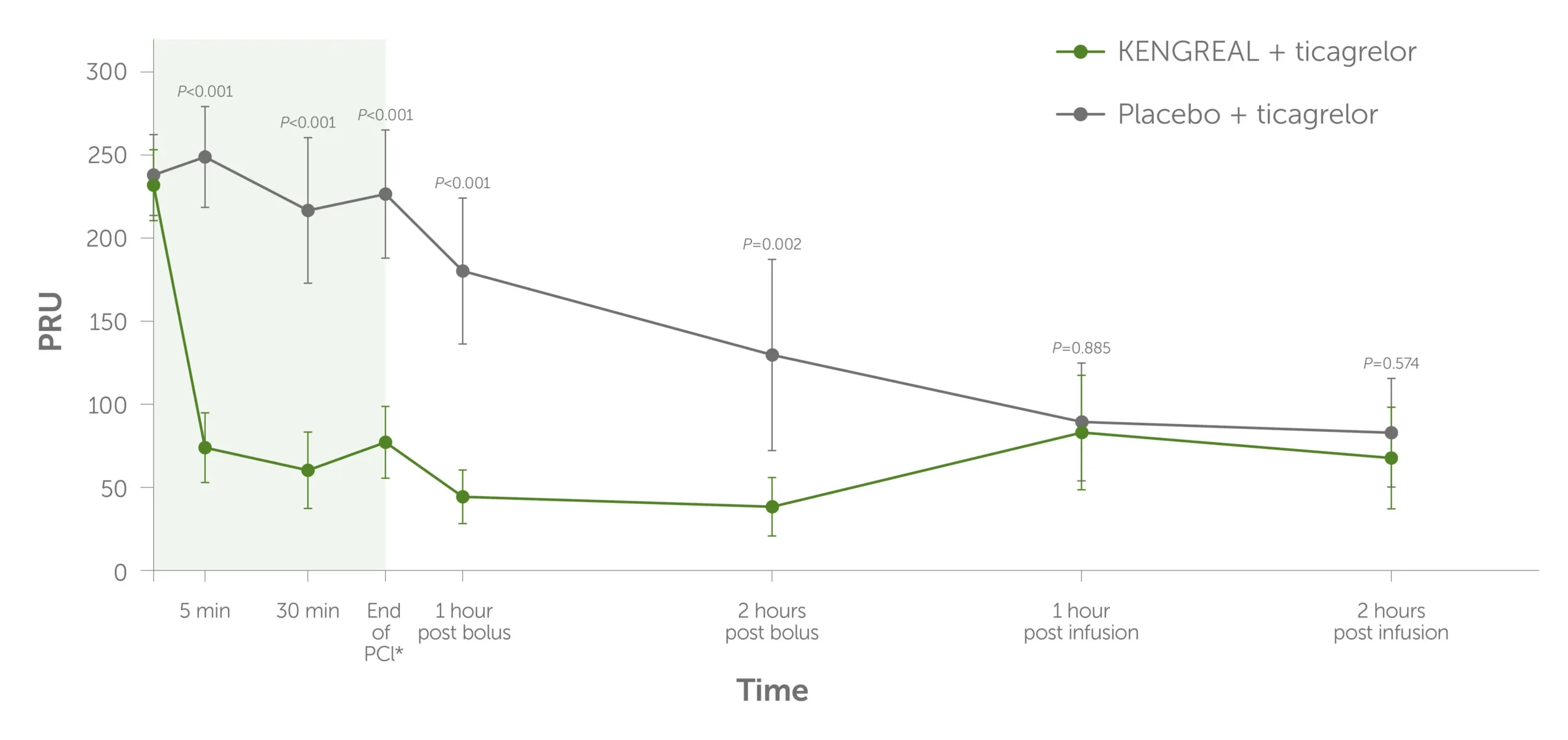
In CANTIC, KENGREAL and crushed ticagrelor were concomitantly administered. KENGREAL plus crushed ticagrelor demonstrated more rapid and potent P2Y12 inhibition than crushed ticagrelor alone. Significant differences were observed as early as 5 minutes post bolus administration.1
- No drug-drug interactions with ticagrelor
- No difference in PRU levels between treatment arms after discontinuation of drug infusion
Platelet inhibition over time as measured by PRU levels1
PD findings need to be interpreted with caution and do not represent adequate evidence of clinical implications. Assessment of clinically relevant endpoints warrant further investigation in an adequately powered clinical trial.
Graph showing platelet inhibition, as measured by the level of P2Y12 reaction units (PRU) during PCI to 2 hours post infusion. KENGREAL + ticagrelor demonstrated numerically lower PRU throughout compared to placebo + ticagrelor.
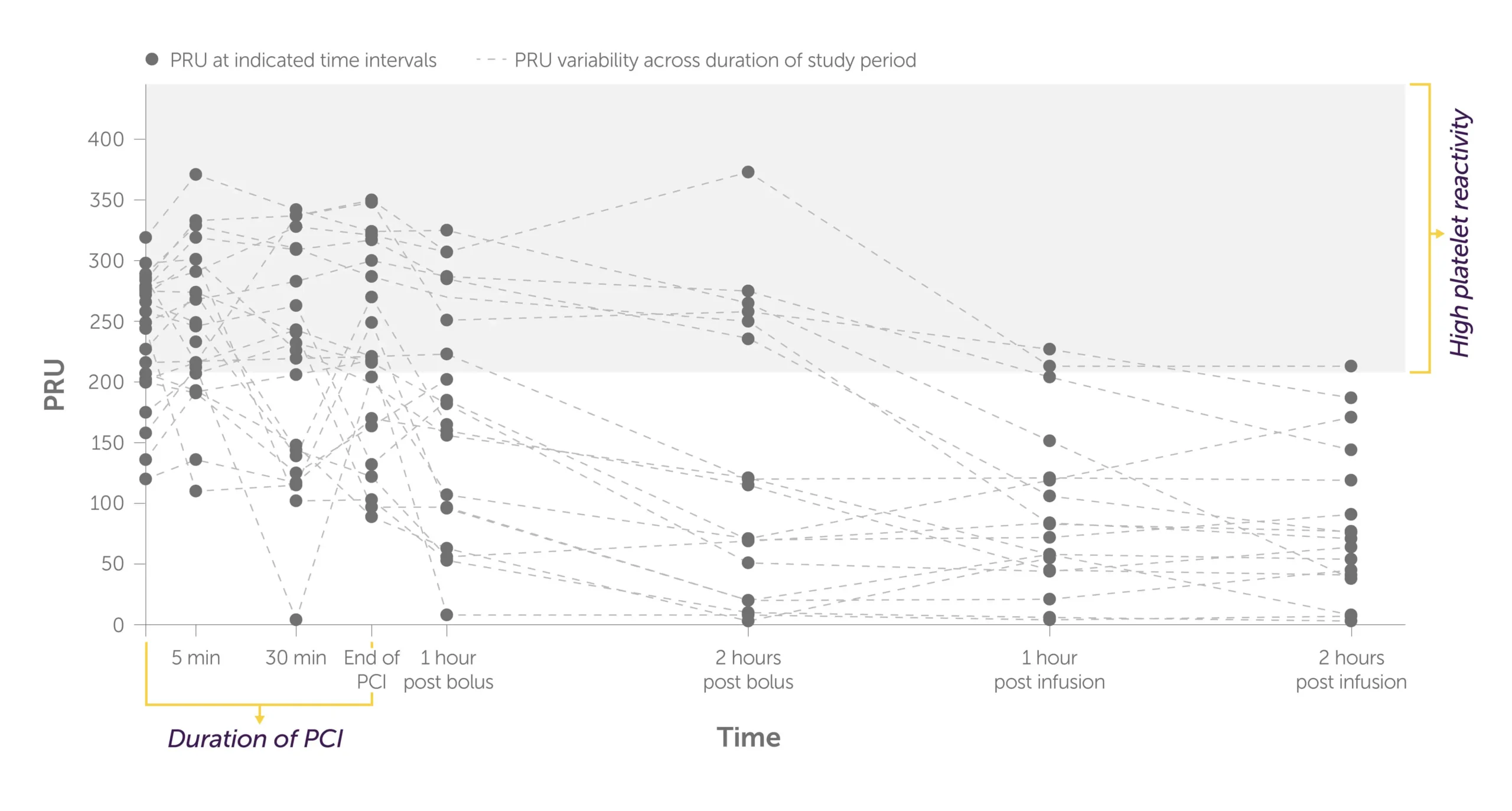
*End of PCI was 41 minutes (range: 21-54 minutes) after KENGREAL/placebo bolus. Values >208 PRU (measured by VerifyNow™ assay) were considered evidence of HPR.1 Error bars indicate 95% confidence intervals.
HPR=high platelet reactivity; PCI=percutaneous coronary intervention; PD=pharmacodynamic; PRU=P2Y12 reaction units; STEMI-ST-elevation myocardial infarction.
See CANTIC study details
Read more about CANTIC, a prospective, randomized, double-blind, placebo-controlled study in STEMI patients undergoing primary PCI (n=50; PD population=44).1
Go to CANTIC PublicationKENGREAL is associated with significantly reduced rates of HPR
Compared with placebo, KENGREAL was also associated with significantly reduced rates of HPR during the duration of drug infusion, which was assessed by measurements of PRU >208 and PRI >50%.1
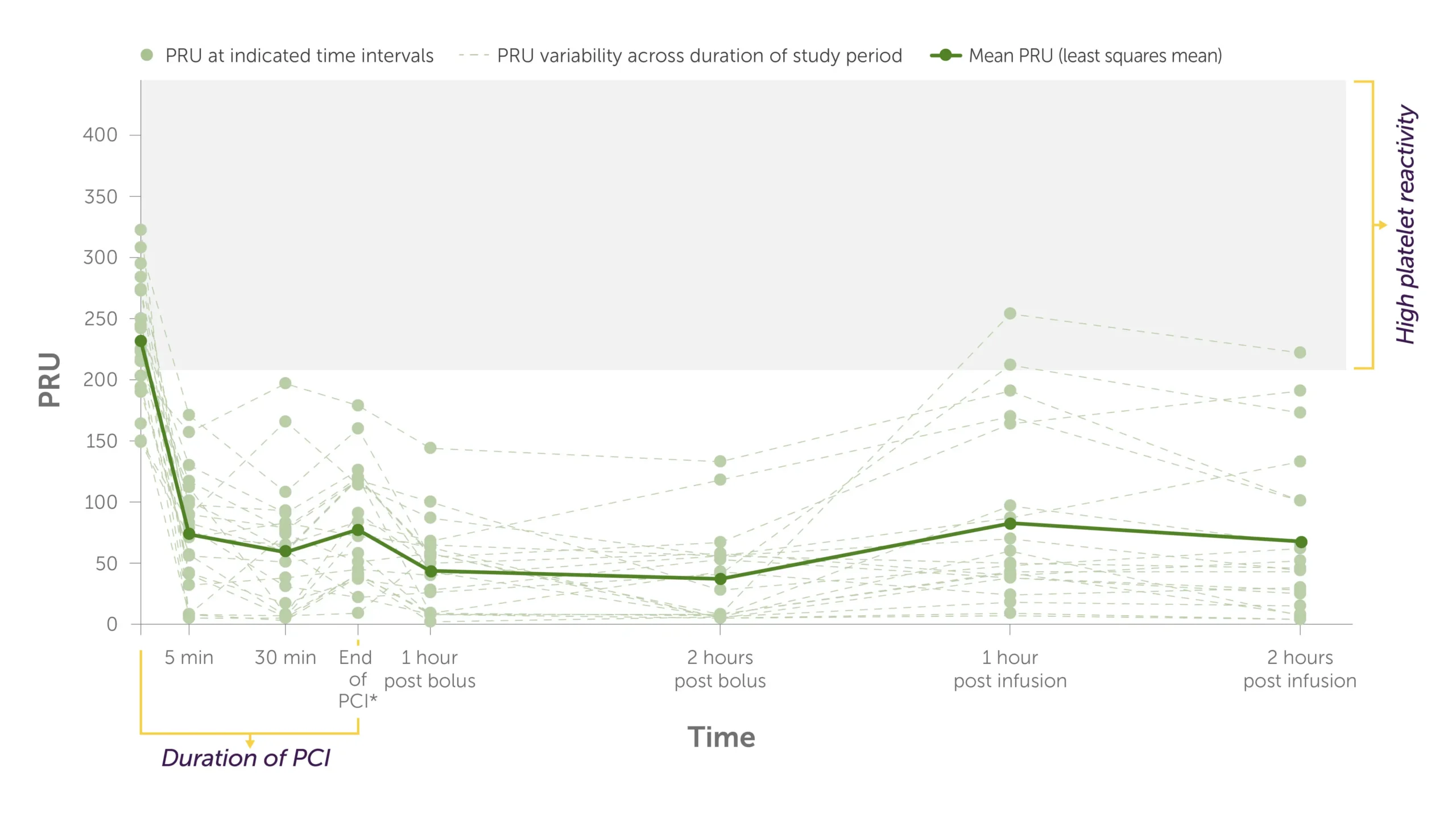
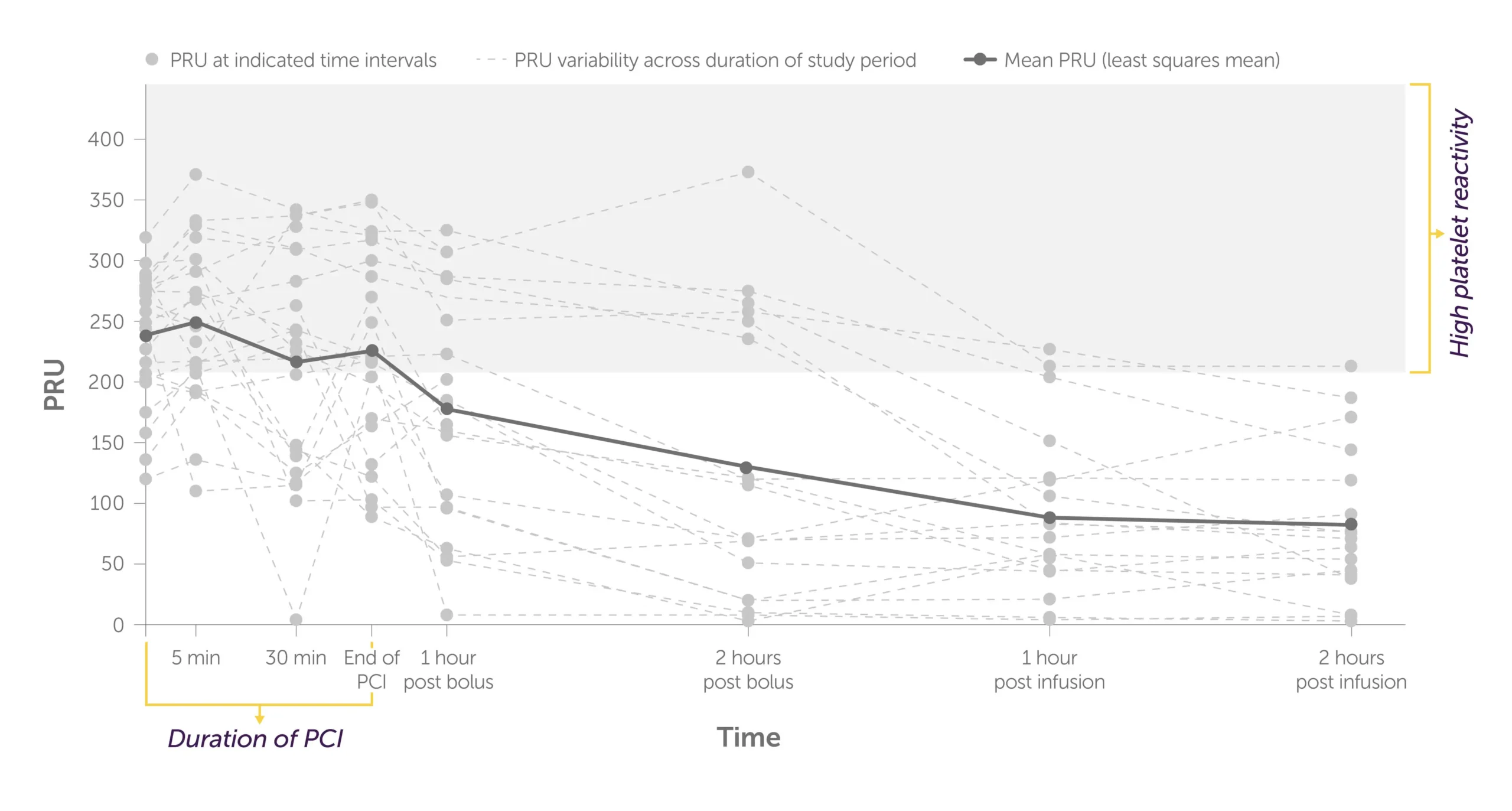
Variability in platelet inhibition
KENGREAL + crushed ticagrelor: Individual PRU values1
PD findings need to be interpreted with caution and do not represent adequate evidence of clinical implications. Assessment of clinically relevant endpoints warrant further investigation in an adequately powered clinical trial.
PD findings need to be interpreted with caution and do not represent adequate evidence of clinical implications. Assessment of clinically relevant endpoints warrant further investigation in an adequately powered clinical trial.
Graph showing the trajectory of the levels of PRU per patient during PCI to 2 hours post infusion with KENGREAL + crushed ticagrelor administered.
All patients in the KENGREAL group achieved a PRU <208 threshold for HPR at the first measurement (5 minutes) and maintained a level below threshold for the duration on the infusion.1
*End of PCI was 41 minutes (range: 21-54 minutes) after KENGREAL/placebo bolus.
PRI=platelet reactivity index.
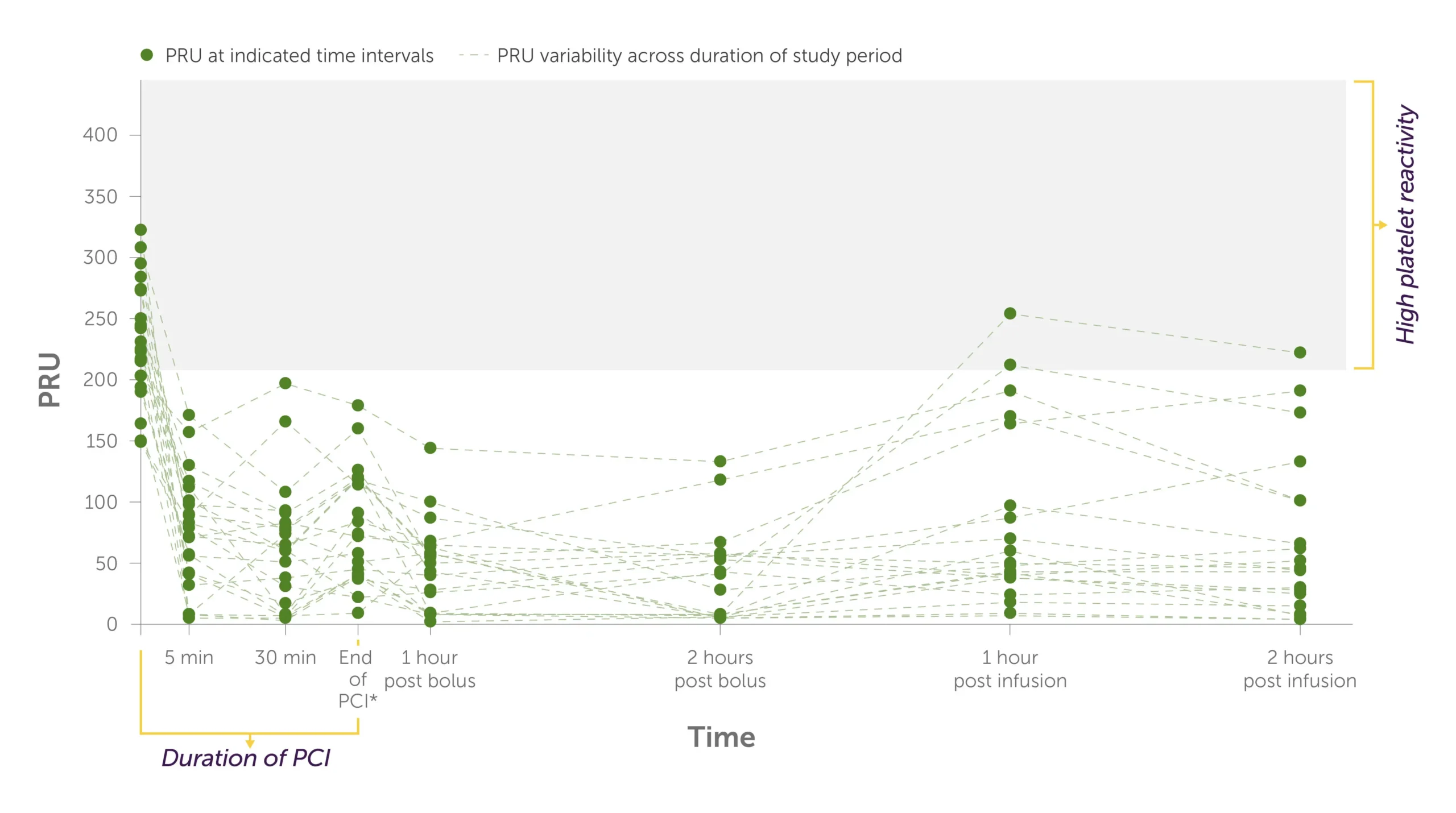
Placebo + crushed ticagrelor1
PD findings need to be interpreted with caution and do not represent adequate evidence of clinical implications. Assessment of clinically relevant endpoints warrant further investigation in an adequately powered clinical trial.
Graph showing the trajectory of the levels of P2Y12 reaction units, or PRU, per patient during PCI to 2 hours post infusion with placebo + crushed ticagrelor administered.
There were higher rates of HPR and more variability in platelet reactivity for individual patients treated with crushed ticagrelor alone.1
All patients were treated with both crushed ticagrelor and fentanyl.1 Fentanyl is known to interfere with absorption of oral P2Y12 inhibitors.2-4 The significance of the interaction on these PD findings is unknown.
Why KENGREAL for patients with STEMI or absorption issues?
See various patient scenarios in which KENGREAL can be applied.
Important Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
References: 1. Franchi F, Rollini F, Rivas A, et al. Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. 2019;139(14):1661-1670. 2. BRILINTA® (ticagrelor) Prescribing Information. 2024. 3. PLAVIX® (clopidogrel) Prescribing Information. 2022. 4. EFFIENT® (prasugrel) Prescribing Information. 2022.
