When to Use KENGREAL® (cangrelor)
See patient scenarios in which KENGREAL may be beneficial.1-9
KENGREAL: Which patients?
Complex coronary anatomy2
- Visible thrombus/thrombotic lesions
- Multi-lesion PCI
- Calcified lesions
- Other angiographic high-risk lesion features
Oral therapy compromised or not feasible3*
- Cardiogenic shock4†
- Out-of-hospital cardiac arrest5†
- Intubation
- Known or potential need for CABG or other surgery immediately after PCI3
- Nausea or vomiting
- Coadministration with fentanyl and/or morphine3,6-8‡
High-risk comorbidities
- Renal impairment or unknown renal status3§
- Peripheral arterial disease9
- Diabetes mellitus1
- Hepatic impairment3§
Patient symptoms will vary; individual clinical evaluation should be done to determine the best course of therapy. Clinical, hemodynamic, anatomical, and procedural elements intricately add to the complexity of PCI procedures, impacting patient outcomes and treatment strategies.
*IV administration with onset of 2 minutes and offset within 1 hour.
†Randomized, controlled trials have not been conducted to evaluate the safety and efficacy of KENGREAL in patients with cardiogenic shock or out-of-hospital cardiac arrest.
‡Opioids are known to interfere with the absorption of oral P2Y12 inhibitors.
§No dosage adjustment required for renal or hepatic impairment.
Why KENGREAL for PCI?

Given its IV administration, KENGREAL can be used in patients who are unconscious or cannot take oral agents3

With KENGREAL, platelet function will recover to baseline within an hour after discontinuation, which may be important for patients who need to move on to surgery3
Case profiles for KENGREAL useII
These hypothetical profiles demonstrate the types of cases in which KENGREAL may be useful.
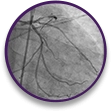
STEMI
case profile
Thrombotic total occlusion of the LAD and evidence of fresh thrombus in a 62-year-old male patient who has acutely received morphine.
STEMI Case Profile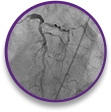
NSTEMI
case profile
Bifurcated lesion involving the circumflex artery, 80% occlusion of the proximal LAD, and evidence of calcification in a 76-year-old female with type 2 diabetes mellitus.
NSTEMI Case Profile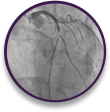
Emergent case with patient absorption issues
Proximal LAD occlusion at the origin of the first diagonal branch in a 76-year-old male with an A1c of 11.2% and CKD stage 3.
Emergent Case ProfileIINot actual patients. Patient symptoms will vary; individual clinical evaluation should be done to determine the best course of therapy.
CABG=coronary artery bypass graft; CKD=chronic kidney disease; IV=intravenous; LAD=left anterior descending artery; NSTEMI=non–ST-elevation myocardial infarction; PCI=percutaneous coronary intervention; STEMI=ST-elevation myocardial infarction.
Important Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
References: 1. Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303-1313. 2. Stone G, Généreux P, Harrington RA, et al. Impact of lesion complexity on peri-procedural adverse events and the benefit of potent intravenous platelet adenosine diphosphate receptor inhibition after percutaneous coronary intervention: core laboratory analysis from 10 854 patients from the CHAMPION PHOENIX trial. Eur Heart J. 2018;39(46):4112-4121. 3. KENGREAL® (cangrelor) Prescribing Information. 2022. 4. Henry TD, Tomey MI, Tamis-Holland JE, et al. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2021;143(15):e815-e829. 5. Tamis-Holland JE, Menon V, Johnson NJ, et al. Cardiac catheterization laboratory management of the comatose adult patient with an out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2024;149(5):e274-e295. 6. BRILINTA® (ticagrelor) Prescribing Information. 2024. 7. EFFIENT® (prasugrel) Prescribing Information. 2022. 8. PLAVIX® (clopidogrel) Prescribing Information. 2022. 9. Gutierrez JA, Harrington RA, Stone GW, et al. Efficacy and safety of cangrelor in patients with peripheral artery disease undergoing percutaneous coronary intervention – Insights from the CHAMPION program. Am Heart J Plus. 2021;9:100043.
